What Is The Charge Of Hydroxide
Kalali
Apr 07, 2025 · 5 min read
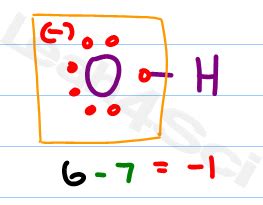
Table of Contents
What is the Charge of Hydroxide? Understanding Hydroxide Ions and Their Role in Chemistry
The hydroxide ion, denoted as OH⁻, is a fundamental chemical species playing a crucial role in numerous chemical reactions and processes. Understanding its charge and behavior is key to comprehending various aspects of chemistry, from acid-base reactions to the properties of aqueous solutions. This comprehensive article will delve deep into the nature of the hydroxide ion, exploring its charge, structure, properties, and its significance in different chemical contexts.
The -1 Charge of the Hydroxide Ion: A Closer Look
The hydroxide ion carries a single negative charge, denoted by the superscript minus sign (⁻). This negative charge arises from the unequal sharing of electrons between the oxygen and hydrogen atoms within the ion.
Electron Configuration and Charge Distribution
Oxygen, with its six valence electrons, strongly attracts the shared electron pair in the O-H bond. This results in a higher electron density around the oxygen atom compared to the hydrogen atom. Essentially, the oxygen atom "takes" an extra electron, leading to the overall negative charge of the hydroxide ion. This unequal electron sharing is a characteristic of polar covalent bonds, and the hydroxide ion is a prime example of this phenomenon.
The Role of Electronegativity
The electronegativity difference between oxygen (highly electronegative) and hydrogen (less electronegative) is the driving force behind the negative charge on the hydroxide ion. Electronegativity refers to an atom's ability to attract electrons within a chemical bond. Oxygen's high electronegativity enables it to pull the shared electrons closer to itself, resulting in a partial negative charge (δ-) on the oxygen and a partial positive charge (δ+) on the hydrogen. This polar nature is crucial in the hydroxide ion's reactivity and interactions with other molecules.
Formation of Hydroxide Ions: Dissociation and Reactions
Hydroxide ions are commonly formed through the dissociation of water molecules and certain bases.
Dissociation of Water: A Dynamic Equilibrium
Water itself undergoes a self-ionization process, where a small fraction of water molecules dissociate into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻):
2H₂O ⇌ H₃O⁺ + OH⁻
This equilibrium is crucial in determining the pH of water and aqueous solutions. The concentration of both hydronium and hydroxide ions are equal in pure water, leading to a neutral pH of 7.
Strong Bases and Hydroxide Ion Production
Strong bases, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH), readily dissociate in water to release hydroxide ions:
NaOH → Na⁺ + OH⁻ KOH → K⁺ + OH⁻
These reactions significantly increase the hydroxide ion concentration in the solution, resulting in an alkaline or basic pH (pH > 7). The concentration of hydroxide ions directly influences the basicity of a solution.
Properties and Reactions of Hydroxide Ions: A Diverse Chemical Landscape
The hydroxide ion's negative charge and its high reactivity contribute to its diverse roles in various chemical processes.
Reactivity with Acids: Neutralization Reactions
Hydroxide ions are strong bases and react readily with acids in neutralization reactions. These reactions involve the combination of hydroxide ions with hydronium ions (or hydrogen ions) to form water:
OH⁻ + H₃O⁺ → 2H₂O
This reaction is highly exothermic, meaning it releases heat. Neutralization reactions are fundamental in acid-base titrations and many other chemical applications.
Formation of Metal Hydroxides: Precipitation Reactions
Hydroxide ions can react with metal cations to form metal hydroxides, some of which are insoluble and precipitate out of solution. For example, the reaction of iron(III) ions with hydroxide ions produces iron(III) hydroxide, a reddish-brown precipitate:
Fe³⁺ + 3OH⁻ → Fe(OH)₃(s)
Precipitation reactions involving hydroxide ions are used in qualitative analysis to identify metal ions.
Hydrolysis Reactions: Changing pH
Hydroxide ions can participate in hydrolysis reactions, where they react with water molecules to affect the solution's pH. For instance, the hydrolysis of certain salts can lead to the production of hydroxide ions, resulting in a basic solution.
Redox Reactions: Less Common but Important
While less prominent than its acid-base reactions, the hydroxide ion can participate in certain redox (reduction-oxidation) reactions. These reactions involve the transfer of electrons, and the hydroxide ion can act as either an electron donor or acceptor depending on the reaction conditions and the other reactants involved.
The Importance of Hydroxide Ions in Various Fields
The hydroxide ion's ubiquitous nature makes it essential in various fields.
Industrial Applications: From Cleaning to Manufacturing
Hydroxide ions are widely used in industrial processes, including:
- Cleaning and sanitation: Hydroxide-containing solutions are common cleaning agents due to their ability to dissolve grease, oils, and other substances.
- Manufacturing: Hydroxide ions are used in various manufacturing processes, such as the production of soaps, detergents, and other chemicals.
- Water treatment: Hydroxide ions play a role in water treatment processes to adjust pH and remove impurities.
Biological Systems: Maintaining pH and Enzyme Function
In biological systems, hydroxide ions contribute to maintaining pH homeostasis, which is crucial for enzyme activity and cellular function. The delicate balance between hydronium and hydroxide ions is essential for the proper functioning of living organisms.
Environmental Science: Understanding Water Quality
Hydroxide ion concentration is a key indicator of water quality. Elevated hydroxide levels can indicate pollution from alkaline sources, while low levels can signal acidic contamination. Monitoring hydroxide levels is crucial for environmental protection and management.
Conclusion: A Fundamental Ion with Diverse Roles
The hydroxide ion, with its characteristic -1 charge, is a fundamental chemical species with a profound impact on chemistry and related fields. Its reactivity in acid-base reactions, its role in forming precipitates, and its contribution to pH regulation are just some examples of its significance. Understanding the charge, properties, and reactions of the hydroxide ion is crucial for comprehending a wide array of chemical and biological processes. From industrial applications to biological systems, its presence and influence are pervasive and undeniably important. Further exploration into its diverse roles continues to unveil its importance in our ever-expanding understanding of the chemical world.
Latest Posts
Latest Posts
-
What Percent Of 2 Is 8
Apr 09, 2025
-
How Many Fluid Ounces In 100 Ml
Apr 09, 2025
-
How Many Cups Of Sour Cream Is 8 Oz
Apr 09, 2025
-
1 Cubic Ft To Square Feet
Apr 09, 2025
-
How Many Gallons Is The Ocean
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Hydroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.