What Is The Difference Between A Reactant And A Product
Kalali
Mar 16, 2025 · 5 min read
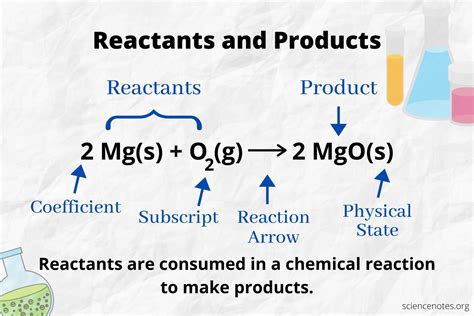
Table of Contents
What's the Difference Between a Reactant and a Product? A Deep Dive into Chemical Reactions
Understanding the difference between reactants and products is fundamental to grasping the core concepts of chemistry. While seemingly simple at first glance, a thorough understanding involves delving into the intricacies of chemical reactions, reaction mechanisms, and the conservation of mass. This article will provide a comprehensive explanation, exploring the definitions, distinguishing features, and practical examples to solidify your understanding.
Defining Reactants and Products
Before diving into the distinctions, let's clearly define each term:
Reactants: The Starting Materials
Reactants are the substances that undergo a chemical change during a reaction. They are the initial ingredients that are consumed or transformed as the reaction progresses. Think of them as the "input" in a chemical process. Reactants are listed on the left-hand side of a chemical equation, separated by plus (+) signs. They are the starting point of any chemical transformation. The properties of the reactants, such as their chemical structure, reactivity, and concentration, significantly influence the rate and outcome of the reaction.
Products: The Result of the Reaction
Products are the substances formed as a result of a chemical reaction. They are the new substances created after the reactants have undergone a chemical change. Consider them the "output" of the chemical process. Products are listed on the right-hand side of a chemical equation, also separated by plus (+) signs. The properties of the products are different from those of the reactants, reflecting the transformation that has occurred. Analyzing the products allows chemists to understand the nature and mechanism of the reaction.
Key Differences Between Reactants and Products
The fundamental difference lies in their roles within a chemical reaction:
| Feature | Reactants | Products |
|---|---|---|
| Role | Starting materials, undergoing change | Substances formed as a result of the change |
| Location in Equation | Left-hand side | Right-hand side |
| State of Change | Consumed or transformed during reaction | Formed as a result of the reaction |
| Properties | Different properties from products | Different properties from reactants |
Visualizing the Transformation: An Analogy
Imagine baking a cake. The reactants are the ingredients: flour, sugar, eggs, butter, etc. The process of baking is the chemical reaction, where the heat and interaction of ingredients cause a transformation. The product is the finished cake – a completely new substance with different properties (taste, texture, appearance) than the individual ingredients.
The Law of Conservation of Mass: A Crucial Principle
The concept of reactants and products is inextricably linked to the Law of Conservation of Mass. This fundamental law of chemistry states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants must equal the total mass of the products. No atoms are lost or gained during the reaction; they are simply rearranged to form new molecules.
This principle is crucial in balancing chemical equations. When writing a chemical equation, you must ensure that the number of atoms of each element is the same on both the reactant and product sides. This ensures that the equation accurately reflects the Law of Conservation of Mass.
Types of Chemical Reactions and Reactant/Product Relationships
Numerous types of chemical reactions exist, each involving specific reactant and product relationships:
1. Synthesis Reactions (Combination Reactions):
In synthesis reactions, two or more reactants combine to form a single product.
Example: 2H₂ (g) + O₂ (g) → 2H₂O (l)
Here, hydrogen gas (H₂) and oxygen gas (O₂) are the reactants, combining to form water (H₂O) as the product.
2. Decomposition Reactions:
Decomposition reactions involve a single reactant breaking down into two or more products.
Example: 2H₂O (l) → 2H₂ (g) + O₂ (g)
Water (H₂O) is the reactant, decomposing into hydrogen gas (H₂) and oxygen gas (O₂) as products. This is the reverse of the synthesis reaction above.
3. Single Displacement Reactions (Substitution Reactions):
In single displacement reactions, one element replaces another element in a compound.
Example: Zn (s) + 2HCl (aq) → ZnCl₂ (aq) + H₂ (g)
Zinc (Zn) replaces hydrogen (H) in hydrochloric acid (HCl), forming zinc chloride (ZnCl₂) and hydrogen gas (H₂) as products.
4. Double Displacement Reactions (Metathesis Reactions):
Double displacement reactions involve the exchange of ions between two compounds.
Example: AgNO₃ (aq) + NaCl (aq) → AgCl (s) + NaNO₃ (aq)
Silver nitrate (AgNO₃) and sodium chloride (NaCl) react, exchanging ions to form silver chloride (AgCl) and sodium nitrate (NaNO₃).
5. Combustion Reactions:
Combustion reactions involve the rapid reaction of a substance with oxygen, typically producing heat and light.
Example: CH₄ (g) + 2O₂ (g) → CO₂ (g) + 2H₂O (l)
Methane (CH₄) reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O).
Identifying Reactants and Products in Chemical Equations
Recognizing reactants and products in a chemical equation is straightforward:
- Reactants are always on the left side of the arrow.
- Products are always on the right side of the arrow.
- The arrow indicates the direction of the reaction. It means "yields" or "produces."
Beyond Simple Reactions: Complex Reactions and Reaction Mechanisms
While the examples above illustrate simple reactions, many chemical processes are far more complex. These reactions may involve multiple steps, intermediate compounds, and reaction mechanisms. Even in these complex scenarios, the fundamental distinction between reactants and products remains valid. The initial substances entering the reaction pathway are still considered reactants, and the final substances formed are the products. The intermediate compounds are simply temporary species formed and consumed during the reaction's progression.
The Importance of Stoichiometry
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. It uses the balanced chemical equation to determine the amounts of reactants needed to produce a certain amount of product, or vice-versa. Understanding stoichiometry is crucial in many chemical applications, including industrial processes, pharmaceutical production, and environmental monitoring.
Conclusion: A Cornerstone of Chemistry
The difference between reactants and products is a cornerstone concept in chemistry. Understanding their roles, the law of conservation of mass, and the various types of chemical reactions is vital for anyone studying or working in this field. By grasping these fundamental ideas, you can better understand how chemical transformations occur, predict reaction outcomes, and apply chemical principles to solve real-world problems. From simple laboratory experiments to complex industrial processes, the interplay between reactants and products remains a central theme in the fascinating world of chemistry. Continuous study and practice are key to mastering this fundamental concept and expanding your knowledge of chemical reactions and their diverse applications.
Latest Posts
Latest Posts
-
Is Sand And Water A Homogeneous Mixture
Mar 17, 2025
-
What Is The Molar Mass Of Co
Mar 17, 2025
-
24 Feet Is How Many Meters
Mar 17, 2025
-
The Property Of Volume Is A Measure Of
Mar 17, 2025
-
Distance From Earth To The Sun In Light Years
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Reactant And A Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
