What Is The Electron Configuration Of Tellurium
Kalali
Apr 01, 2025 · 6 min read
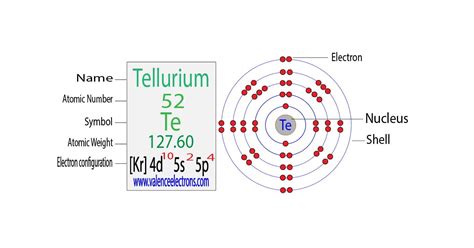
Table of Contents
What is the Electron Configuration of Tellurium? A Deep Dive into Atomic Structure
Tellurium, a metalloid element with the symbol Te and atomic number 52, holds a fascinating place in the periodic table. Understanding its electron configuration is key to comprehending its chemical properties and behavior. This comprehensive guide will explore the electron configuration of tellurium, examining its underlying principles, variations, and implications. We'll also delve into the nuances of its electronic structure and how it contributes to Tellurium's unique characteristics.
Understanding Electron Configuration
Before diving into Tellurium's specific configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. This arrangement is governed by the principles of quantum mechanics, which dictate the probabilities of finding electrons in specific regions of space around the nucleus. Knowing an element's electron configuration is crucial because it directly influences its chemical reactivity, bonding behavior, and physical properties.
The electron configuration is usually expressed using a notation that specifies the principal energy level (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For instance, 1s² indicates two electrons in the 1s subshell, the lowest energy level.
Deriving Tellurium's Electron Configuration
Tellurium (Te) has an atomic number of 52, meaning it possesses 52 protons and, in its neutral state, 52 electrons. To determine its electron configuration, we follow the Aufbau principle, filling orbitals in order of increasing energy. The order is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... However, exceptions exist due to subtle energy level variations.
Following this principle, the expected electron configuration of Tellurium would be:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁴
This configuration indicates that Tellurium's 52 electrons are distributed across various energy levels and subshells. Let's break it down further:
- 1s²: Two electrons occupy the lowest energy level (n=1) in the s subshell.
- 2s² 2p⁶: Eight electrons fill the second energy level (n=2), with two in the s subshell and six in the p subshell.
- 3s² 3p⁶: Another eight electrons fill the third energy level (n=3), similarly distributed.
- 4s² 3d¹⁰ 4p⁶: Eighteen electrons occupy the fourth energy level (n=4), including two in the s, ten in the d, and six in the p subshell. Note that the 3d subshell fills after the 4s.
- 5s² 4d¹⁰ 5p⁴: The remaining sixteen electrons are in the fifth energy level (n=5), with two in the s, ten in the d, and four in the p subshell.
This detailed breakdown showcases the systematic filling of orbitals according to the Aufbau principle.
Noble Gas Configuration and Tellurium
A simplified way to represent Tellurium's electron configuration utilizes the noble gas configuration. Noble gases are characterized by their exceptionally stable electron configurations, with completely filled valence shells. Krypton (Kr), with an atomic number of 36, is the noble gas preceding Tellurium. Therefore, we can express Tellurium's configuration as:
[Kr] 5s² 4d¹⁰ 5p⁴
This notation means that Tellurium has the same electron configuration as Krypton, plus the additional sixteen electrons in the 5s, 4d, and 5p subshells. This shorthand notation is commonly used for its brevity and clarity.
Valence Electrons and Chemical Reactivity
The outermost electrons, residing in the highest energy level, are known as valence electrons. These electrons are responsible for the element's chemical behavior and bonding capabilities. In Tellurium's case, the valence electrons are those in the 5s and 5p subshells, totaling six electrons (5s² 5p⁴). This electron configuration explains Tellurium's tendency to form covalent bonds and its ability to exist in various oxidation states, reflecting the flexibility of its valence electrons. The four unpaired electrons in the 5p subshell contribute significantly to its chemical reactivity.
Exceptions and Subtleties in Electron Configuration
While the Aufbau principle provides a good general guideline, exceptions do arise. These exceptions stem from subtle energy differences between subshells and the influence of electron-electron interactions. While Tellurium's electron configuration adheres to the standard Aufbau principle, other elements might show variations, particularly in the d and f subshells. These variations are often explained by the relative stability gained through half-filled or completely filled subshells.
Tellurium's Physical and Chemical Properties: An Electron Configuration Perspective
Tellurium's electron configuration directly impacts its physical and chemical properties. Its metalloid nature, a blend of metallic and non-metallic characteristics, arises from its incompletely filled valence shell. This partially filled p subshell allows Tellurium to exhibit both metallic (e.g., electrical conductivity) and non-metallic (e.g., formation of covalent bonds) properties, distinguishing it from true metals and nonmetals.
- Conductivity: The relatively loosely held valence electrons allow for some electrical conductivity, although less than typical metals.
- Covalent Bonding: The tendency to share electrons with other atoms allows for the formation of various covalent compounds.
- Oxidation States: Tellurium can exhibit multiple oxidation states, ranging from -2 to +6, reflecting its ability to gain or lose electrons to achieve a more stable configuration.
Applications of Tellurium: A Link to its Electronic Structure
Understanding Tellurium's electron configuration is crucial to understanding its applications. Its unique properties are exploited in various technological fields:
- Solar Cells: Tellurium's semiconductor properties make it suitable for use in solar cells, converting sunlight into electricity. The electronic structure influences its band gap and efficiency in this application.
- Metal Alloys: Tellurium's addition to certain metal alloys can improve their machinability and other physical properties. The interaction between its valence electrons and those of the metal atoms is key here.
- Rubber Vulcanization: Tellurium compounds are used in rubber vulcanization, impacting the final product's properties. The electron configuration plays a role in its ability to form cross-links in the rubber polymer.
- Other Applications: Tellurium finds applications in various other specialized fields, from metallurgy to the production of certain catalysts, all stemming from its unique electronic structure.
Conclusion: The Significance of Tellurium's Electron Configuration
Tellurium's electron configuration, [Kr] 5s² 4d¹⁰ 5p⁴, provides a fundamental understanding of its chemical and physical properties. Its incompletely filled valence shell and the relative ease with which it can gain or lose electrons contribute to its ability to form various compounds, its semi-conducting behavior, and its utility in diverse technological applications. By understanding its electronic structure, we can better appreciate Tellurium's multifaceted nature and its important contributions to various scientific and industrial sectors. Further exploration into the nuances of its electronic interactions will continue to enhance our understanding and lead to innovative applications of this fascinating metalloid. The knowledge presented here serves as a solid foundation for more advanced studies in Tellurium's chemistry and its potential for future advancements.
Latest Posts
Latest Posts
-
Cuantos Son 40 Grados Fahrenheit En Centigrados
Apr 02, 2025
-
Differentiate Between Extrusive And Intrusive Rocks
Apr 02, 2025
-
How Do You Write A Congruence Statement
Apr 02, 2025
-
What Is The Relationship Between Electron Affinity And Atomic Radius
Apr 02, 2025
-
What Is 44 50 As A Percent
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Tellurium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
