What Is The Molar Mass Of Aucl3
Kalali
Mar 14, 2025 · 5 min read
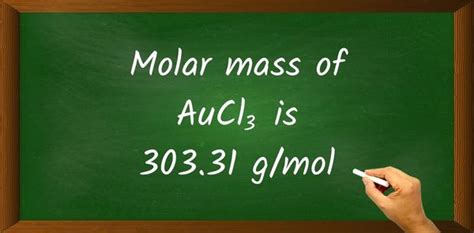
Table of Contents
What is the Molar Mass of AuCl₃? A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This article delves into the specifics of calculating the molar mass of gold(III) chloride (AuCl₃), explaining the process step-by-step and highlighting its importance in different chemical contexts. We'll also explore related concepts and practical applications.
Understanding Molar Mass
Before diving into the calculation for AuCl₃, let's establish a clear understanding of molar mass. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of elementary entities, be it atoms, molecules, ions, or other specified particles. The molar mass is typically expressed in grams per mole (g/mol).
The molar mass of an element is numerically equal to its atomic weight (relative atomic mass) found on the periodic table. For compounds, the molar mass is the sum of the molar masses of all the atoms in the chemical formula.
Calculating the Molar Mass of AuCl₃
Gold(III) chloride, AuCl₃, is an ionic compound consisting of one gold(III) ion (Au³⁺) and three chloride ions (Cl⁻). To calculate its molar mass, we need the atomic masses of gold (Au) and chlorine (Cl). These can be found on a periodic table.
- Atomic mass of Gold (Au): Approximately 196.97 g/mol
- Atomic mass of Chlorine (Cl): Approximately 35.45 g/mol
Now, let's perform the calculation:
Molar mass of AuCl₃ = (Molar mass of Au) + 3 * (Molar mass of Cl)
Molar mass of AuCl₃ = 196.97 g/mol + 3 * 35.45 g/mol
Molar mass of AuCl₃ = 196.97 g/mol + 106.35 g/mol
Molar mass of AuCl₃ ≈ 303.32 g/mol
Therefore, the molar mass of AuCl₃ is approximately 303.32 grams per mole. Remember that slight variations might occur depending on the source of atomic mass data used.
Significance of Molar Mass in Chemical Calculations
The molar mass of AuCl₃, and other compounds, is a cornerstone for numerous calculations in chemistry, including:
1. Stoichiometric Calculations:
Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass allows us to convert between mass and moles, which is essential for balancing equations and determining the amounts of substances involved in a reaction. For example, if we want to know how many grams of AuCl₃ are needed to produce a certain amount of a product in a reaction, the molar mass is crucial for this conversion.
2. Concentration Calculations:
Molarity (mol/L), a common unit of concentration, represents the number of moles of solute per liter of solution. To prepare a solution of a specific molarity, the molar mass is needed to accurately weigh out the required amount of AuCl₃.
3. Determining Empirical and Molecular Formulas:
Molar mass plays a vital role in determining the empirical and molecular formulas of unknown compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms of each element in a molecule. By combining experimental data (like percentage composition) with the molar mass, chemists can deduce the molecular formula.
4. Gas Law Calculations:
The ideal gas law (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). The molar mass is essential to convert between mass and moles when using this law to describe the behavior of gases.
5. Thermochemical Calculations:
Many thermochemical calculations, such as determining enthalpy changes (ΔH) in reactions, require knowing the molar mass of reactants and products to express the heat transferred per mole of substance.
Properties and Applications of AuCl₃
Gold(III) chloride is a significant compound with several notable properties and applications:
-
Appearance: It exists as a red-brown crystalline solid.
-
Reactivity: It's a highly reactive compound, readily dissolving in water to form a yellow solution. It reacts with a variety of other substances, acting as a strong oxidizing agent.
-
Applications: Its primary use is in the purification of gold and in various gold plating processes. It also finds applications in photography, medicine (though less common now due to toxicity concerns), and as a catalyst in certain organic reactions.
Safety Precautions when Handling AuCl₃
AuCl₃, like many gold compounds, requires careful handling due to its potential toxicity. Direct contact with skin or inhalation of dust should be avoided. Appropriate personal protective equipment (PPE), including gloves, eye protection, and a respirator, should always be used when working with AuCl₃. Proper disposal procedures should also be followed to minimize environmental impact.
Advanced Concepts and Further Exploration
This section briefly touches upon more advanced concepts related to molar mass and AuCl₃:
-
Isotopes and Atomic Mass: The atomic masses used in our calculation are weighted averages based on the natural abundance of different isotopes of gold and chlorine. If we were dealing with a specific isotopic composition, the molar mass would be slightly different.
-
Hydrates: Gold(III) chloride can form hydrates, meaning water molecules are incorporated into its crystal structure. The presence of water molecules would increase the molar mass.
-
Complex Ions: AuCl₃ can participate in the formation of complex ions, where it acts as a ligand, coordinating with metal ions. Understanding the stoichiometry of these complexes is important for their applications and further calculations.
Conclusion
The molar mass of AuCl₃ is approximately 303.32 g/mol. Understanding this fundamental concept is crucial for various chemical calculations and analyses, from simple stoichiometry problems to more complex applications in different areas of chemistry. Remember always to prioritize safety when handling AuCl₃ and other chemicals. This detailed explanation serves as a comprehensive guide for calculating molar masses and understanding their significant role in chemistry. Through a thorough grasp of this fundamental concept, one can navigate complex chemical problems with confidence.
Latest Posts
Latest Posts
-
Equation Relating Electric Field And Voltage
Mar 14, 2025
-
What Is The Percentage Of 9 12
Mar 14, 2025
-
Force Acting Over A Distance Is The Definition Of
Mar 14, 2025
-
How Many Meters Is 5 Km
Mar 14, 2025
-
Which Characteristic Is Given By The Angular Momentum Quantum Number
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Aucl3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
