What Type Of Ions Do Transition Metals Form
Kalali
Mar 31, 2025 · 6 min read
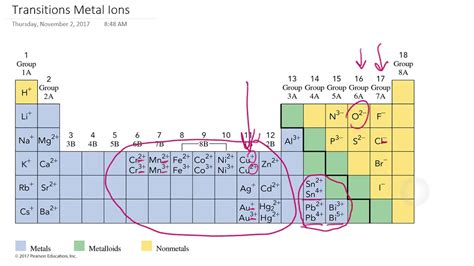
Table of Contents
What Types of Ions Do Transition Metals Form?
Transition metals, those fascinating elements nestled in the d-block of the periodic table, are renowned for their diverse and often captivating chemistry. Unlike the alkali metals or alkaline earth metals, which predictably form ions with a single, easily determined charge, transition metals exhibit a much richer variety of ionic forms. Understanding the types of ions they form is crucial to grasping their diverse applications in catalysis, pigments, and countless other fields. This article delves deep into the fascinating world of transition metal ions, exploring the factors influencing their charge, common oxidation states, and the unique properties that arise from their variable valency.
The D-Block Dilemma: Why Variable Oxidation States?
The key to understanding the diverse ion formation of transition metals lies within their electronic configuration. Unlike elements in the s-block and p-block, where valence electrons are primarily located in the outermost s and p orbitals, transition metals have valence electrons in both the outermost s orbital and the penultimate d orbital. This proximity and the relatively similar energy levels of these orbitals allow for a much greater flexibility in electron loss during ion formation.
Variable Oxidation States: A Defining Characteristic
The term "variable oxidation state" or "variable valency" is central to understanding transition metal chemistry. It refers to the ability of a transition metal atom to lose a variable number of electrons to form ions with different charges. For example, iron (Fe) can readily exist as Fe²⁺ (ferrous) and Fe³⁺ (ferric) ions, while manganese (Mn) can exhibit oxidation states ranging from +2 to +7. This contrasts sharply with the alkali metals, which almost exclusively form +1 ions, and the alkaline earth metals, which typically form +2 ions.
Factors Influencing Oxidation State:
Several factors influence the specific oxidation state a transition metal will adopt in a given compound or complex:
-
Ligand Field Stabilization Energy (LFSE): This is a crucial concept in coordination chemistry. Ligands, the atoms or molecules surrounding the central metal ion, affect the energy levels of the d orbitals. The stabilization energy gained by specific electron configurations in the presence of ligands dictates the preferred oxidation state.
-
Ionic Radius and Charge Density: The size and charge density of the metal ion play a significant role. Smaller, highly charged ions tend to favor higher oxidation states due to increased polarization effects on ligands.
-
Electronegativity of Ligands: The electronegativity of the ligands bonded to the transition metal affects the electron density around the metal, influencing the stability of different oxidation states. Strongly electronegative ligands can stabilize higher oxidation states by drawing electron density away from the metal.
-
Crystal Field Effects: In solid-state compounds, the arrangement of ions in the crystal lattice (crystal field) significantly affects the energy levels of d orbitals and the stability of various oxidation states.
Common Oxidation States of Transition Metals: A Closer Look
Let's examine some specific examples to illustrate the range of oxidation states exhibited by common transition metals. Note that this is not an exhaustive list but serves to highlight the diversity:
Iron (Fe):
Iron is perhaps one of the most well-known transition metals, showcasing two prevalent oxidation states:
-
Fe²⁺ (Ferrous): Found in compounds like ferrous sulfate (FeSO₄), this ion is characterized by a pale green color in aqueous solution.
-
Fe³⁺ (Ferric): Present in compounds like ferric oxide (Fe₂O₃, rust), this ion exhibits a yellowish-brown or reddish-brown color.
The relative stability of Fe²⁺ and Fe³⁺ depends heavily on the environment; oxygen often facilitates oxidation to the Fe³⁺ state.
Manganese (Mn):
Manganese exhibits a remarkably wide range of oxidation states:
-
Mn²⁺: A pale pink ion found in manganese(II) sulfate.
-
Mn³⁺: Less common and generally less stable than Mn²⁺.
-
Mn⁴⁺: Found in manganese dioxide (MnO₂), a dark brown solid used in batteries and as a catalyst.
-
Mn⁷⁺: A highly oxidized state, present in the permanganate ion (MnO₄⁻), a strong oxidizing agent with an intense purple color.
Chromium (Cr):
Chromium shows several important oxidation states:
-
Cr²⁺: A blue ion, relatively easily oxidized.
-
Cr³⁺: A common and relatively stable oxidation state, found in chromium(III) oxide (Cr₂O₃), a green pigment.
-
Cr⁶⁺: Exists as the chromate (CrO₄²⁻) and dichromate (Cr₂O₇²⁻) ions, both powerful oxidizing agents with characteristic yellow and orange colors, respectively.
Copper (Cu):
Copper demonstrates two prominent oxidation states:
-
Cu⁺ (Cuprous): A less common oxidation state, present in compounds like cuprous oxide (Cu₂O), a red solid.
-
Cu²⁺ (Cupric): A more stable and commonly observed oxidation state, found in compounds like copper(II) sulfate, which forms blue solutions.
The relative stability of Cu⁺ and Cu²⁺ is sensitive to the ligand environment and the presence of oxidizing or reducing agents.
Applications of Transition Metal Ions: A Diverse Landscape
The diverse oxidation states of transition metals translate into a vast array of applications. These include:
-
Catalysis: Many transition metal ions act as catalysts in chemical reactions, lowering activation energies and speeding up reaction rates. This is crucial in industrial processes and biological systems. For example, iron plays a vital role in enzymes like hemoglobin and cytochrome c, facilitating oxygen transport and electron transfer.
-
Pigments: The vibrant colors of many transition metal ions are exploited in the production of pigments for paints, inks, and ceramics. For instance, chromium(III) oxide (Cr₂O₃) is used as a green pigment, while cobalt(II) oxide (CoO) provides blue hues.
-
Magnetic Materials: Some transition metal compounds exhibit magnetic properties, making them valuable in technologies like data storage and magnetic resonance imaging (MRI). Iron, nickel, and cobalt are key components in many magnetic alloys.
-
Batteries: Transition metal oxides and other compounds are used in various battery systems, providing efficient energy storage. Manganese dioxide (MnO₂) and lithium cobalt oxide (LiCoO₂) are examples of materials used in commercially available batteries.
Beyond Simple Ions: Coordination Complexes
The discussion so far has focused primarily on simple transition metal ions. However, the majority of transition metal chemistry involves coordination complexes – compounds where the central transition metal ion is surrounded by ligands. These ligands can influence the oxidation state of the metal and impart unique properties to the complex.
The formation of coordination complexes is deeply tied to the variable oxidation states of transition metals. Different ligands can stabilize different oxidation states, leading to a rich tapestry of coordination chemistry. Factors like ligand field strength, steric effects, and the nature of the metal ion all play significant roles in determining the stability and properties of these complexes.
Conclusion: A World of Complexity and Versatility
The types of ions formed by transition metals are far from simple or predictable. Their variable oxidation states, a consequence of their electronic configuration and influenced by a complex interplay of factors, provide a foundation for their remarkable diversity in chemical behavior and wide range of applications. From vibrant pigments to essential biological molecules and high-tech materials, the world of transition metal ions continues to fascinate and inspire scientific exploration and technological advancement. Further research continues to unravel the subtleties of transition metal chemistry, revealing new insights into their behavior and expanding their applications in various fields. The journey into understanding these elements is far from over, ensuring a continued fascination and importance in the years to come.
Latest Posts
Latest Posts
-
What Is The Percent Of 20 Out Of 25
Apr 02, 2025
-
Where Is The North Magnetic Pole Of This Current Loop
Apr 02, 2025
-
Magnesium Metal Or Nonmetal Or Metalloid
Apr 02, 2025
-
How Many Ounces Are In 125 Ml
Apr 02, 2025
-
What Is 10 Out Of 15
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Ions Do Transition Metals Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
