What Type Of Mixture Is Oil And Water
Kalali
Mar 27, 2025 · 6 min read
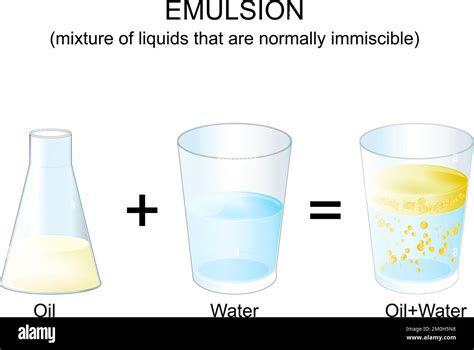
Table of Contents
What Type of Mixture Is Oil and Water? Understanding Immiscible Liquids
Oil and water. The classic example of two substances that simply don't mix. But why don't they mix? Understanding the nature of this seemingly simple interaction delves into the fascinating world of chemistry and the properties of liquids. This comprehensive guide will explore the reasons behind oil and water's incompatibility, examining the scientific principles involved and detailing the type of mixture they form. We'll also look at practical applications and implications of their immiscibility.
The Science Behind Immiscibility: Polarity and Intermolecular Forces
The key to understanding why oil and water don't mix lies in the concept of polarity. Molecules possess a property called polarity, which describes the distribution of electrical charge within the molecule. Water (H₂O) is a polar molecule. This means that the oxygen atom carries a slightly negative charge, while the hydrogen atoms carry a slightly positive charge. This uneven distribution of charge creates a dipole moment. Think of it like a tiny magnet with a positive and negative end.
Oil, on the other hand, is typically composed of nonpolar molecules. These molecules have an even distribution of charge, meaning there's no significant positive or negative region. Examples include hydrocarbons like those found in petroleum-based oils.
This difference in polarity directly affects the intermolecular forces – the forces of attraction between molecules. Polar molecules, like water, are attracted to each other through strong hydrogen bonds, a type of dipole-dipole interaction. These hydrogen bonds are relatively strong, leading to high surface tension and cohesion. Nonpolar molecules, like those in oil, interact primarily through weaker London dispersion forces.
Because water molecules strongly attract each other, they tend to stick together, forming a cohesive network. When oil is introduced, the nonpolar oil molecules are unable to effectively interact with the polar water molecules. The weak London dispersion forces between oil molecules and water molecules are not strong enough to overcome the strong hydrogen bonds between water molecules. This results in the oil molecules being repelled by the water molecules, leading to immiscibility.
Oil and Water: A Heterogeneous Mixture
The combination of oil and water is classified as a heterogeneous mixture. A heterogeneous mixture is one in which the components are not uniformly distributed throughout the mixture. You can easily see the distinct layers of oil and water when they are combined, unlike a homogeneous mixture where the components are evenly dispersed at a molecular level (like saltwater).
Several factors contribute to the heterogeneous nature of the oil-water mixture:
-
Density Difference: Oil typically has a lower density than water, causing it to float on top. This density difference further emphasizes the distinct separation of the two components.
-
Lack of Solubility: The inability of oil and water molecules to interact effectively prevents them from dissolving into each other. Solubility is the ability of one substance to dissolve in another. Oil's insolubility in water, and vice-versa, is a direct consequence of their differing polarities.
-
Phase Separation: The two substances remain in separate phases – the oil remains a liquid phase separate from the water liquid phase. This phase separation is a characteristic feature of heterogeneous mixtures.
Emulsions: A Temporary Suspension
While oil and water are immiscible under normal conditions, they can form a temporary suspension known as an emulsion with the help of an emulsifier. An emulsifier is a substance that stabilizes an emulsion by reducing the interfacial tension between the oil and water phases. Emulsifiers contain both polar and nonpolar regions, allowing them to interact with both oil and water molecules. This creates a bridge, allowing the oil and water to remain suspended in smaller droplets within each other, at least for a period of time.
Examples of emulsifiers include:
-
Soap: Soap molecules have a hydrophilic (water-loving) head and a hydrophobic (water-fearing) tail. The hydrophilic head interacts with water, while the hydrophobic tail interacts with oil, allowing for the dispersion of oil in water.
-
Lecithin: Found in egg yolks and soybeans, lecithin is a natural emulsifier commonly used in food processing.
-
Other Surfactants: Many other chemicals act as surfactants, reducing surface tension and allowing for the formation of emulsions.
Even with an emulsifier, emulsions are not stable indefinitely. Over time, the oil and water phases will tend to separate again unless the emulsion is constantly agitated.
Practical Implications and Applications
The immiscibility of oil and water has significant implications in various fields:
1. Environmental Science: Oil spills in water bodies are a major environmental concern because of the difficulty in cleaning them up. The immiscibility of oil and water prevents the oil from readily dissolving, resulting in persistent pollution. Cleanup efforts often involve using emulsifiers or absorbent materials to remove the oil.
2. Food Industry: The creation of emulsions is crucial in food science. Many food products, such as mayonnaise, salad dressings, and milk, are emulsions stabilized by emulsifiers. Understanding the properties of these emulsions is essential for creating stable and palatable food products.
3. Pharmaceutical Industry: Emulsions are used in many pharmaceutical formulations to deliver drugs. The ability to control the size and stability of these emulsions is important for drug delivery efficiency.
4. Chemical Engineering: The separation of oil and water mixtures is a common problem in chemical processes. Different separation techniques, such as decantation, centrifugation, and filtration, are used depending on the specific requirements.
5. Everyday Life: The simple act of washing dishes relies on the principles of polarity and immiscibility. Soap acts as an emulsifier, allowing the grease (oil) to be suspended in water, making it easier to remove.
Misconceptions and Clarifications
Several common misconceptions surround the oil and water mixture:
-
"Oil dissolves in water": This is incorrect. Oil does not dissolve in water. While a small amount of oil might appear to temporarily disperse, it doesn't dissolve at a molecular level; instead, it forms tiny droplets suspended within the water. True dissolution involves the breaking down of molecules into individual ions or molecules surrounded by solvent molecules.
-
"Mixing vigorously will combine oil and water": Vigorous mixing can create a temporary emulsion, but it does not truly mix the oil and water at a molecular level. Given enough time, the two substances will always separate into distinct layers.
Conclusion: Understanding the Fundamentals of Immiscibility
The seemingly simple interaction of oil and water provides a valuable illustration of the fundamental principles of chemistry. Their immiscibility, stemming from the difference in polarity and the resulting intermolecular forces, has far-reaching consequences in various aspects of science, technology, and everyday life. Understanding the concept of polarity, intermolecular forces, and the formation of emulsions is critical for tackling environmental issues, developing innovative food and pharmaceutical products, and solving practical problems in various engineering fields. The next time you see oil and water separate, remember the intricate chemical dance that dictates their behavior and the numerous applications that depend on this fundamental property. From the macroscopic separation of layers to the microscopic interaction of molecules, the oil-water dichotomy offers a compelling lens through which to explore the world of chemistry.
Latest Posts
Latest Posts
-
Is Carbon Metal Or Non Metal
Mar 30, 2025
-
Walking On Hot Sand Conduction Or Convection
Mar 30, 2025
-
What Is The Division Of The Cytoplasm Called
Mar 30, 2025
-
Is Ductility A Physical Or Chemical Property
Mar 30, 2025
-
Lowest Common Factor Of 3 And 4
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Mixture Is Oil And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
