Which Chemical Equation Represents A Double Replacement Reaction
Kalali
Mar 26, 2025 · 6 min read
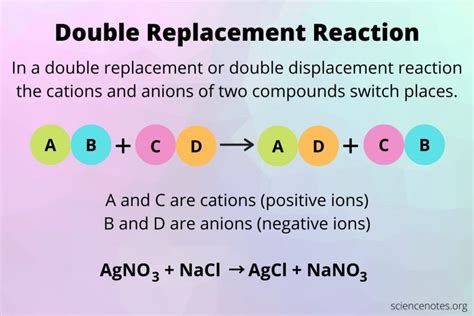
Table of Contents
Which Chemical Equation Represents a Double Replacement Reaction?
Double replacement reactions, also known as double displacement reactions or metathesis reactions, are a common type of chemical reaction where two compounds exchange ions or groups of atoms to form two new compounds. Understanding how to identify these reactions is crucial in chemistry, and this article will delve deep into the characteristics of double replacement reactions, exploring various examples and explaining how to identify them through their chemical equations. We'll also touch upon the conditions that favor these reactions and some common applications.
Understanding Double Replacement Reactions
At the heart of a double replacement reaction lies the exchange of cations (positively charged ions) and anions (negatively charged ions) between two ionic compounds. The general form of the equation is:
AX + BY → AY + BX
Where:
- A and B represent cations.
- X and Y represent anions.
This exchange results in the formation of two new compounds, AY and BX. Crucially, the driving force behind these reactions is often the formation of a precipitate (a solid that forms from a solution), a gas, or water. If none of these products are formed, the reaction may not proceed, or it might reach equilibrium without significant changes in the concentrations of reactants and products.
Identifying the Characteristics of a Double Replacement Reaction
Several key features help distinguish double replacement reactions from other reaction types:
- Ionic Compounds as Reactants: The reactants are typically ionic compounds, meaning they are composed of positively and negatively charged ions held together by electrostatic forces.
- Ion Exchange: The core process involves the swapping of cations and anions between the two reactant compounds.
- Formation of a Precipitate: A common indication of a double replacement reaction is the formation of a solid precipitate, which is often visible as a cloudy or solid substance forming in the solution. Solubility rules are essential here to predict whether a precipitate will form.
- Formation of a Gas: Another indicator is the production of a gas, such as carbon dioxide (CO2) or hydrogen sulfide (H2S). The gas will often bubble out of the solution.
- Formation of Water: The formation of water (H2O) as a product is a strong indicator, as the reaction of an acid and a base (neutralization reaction) is a specific type of double replacement reaction.
Examples of Chemical Equations Representing Double Replacement Reactions
Let's analyze several examples to solidify our understanding:
1. Precipitation Reaction:
Consider the reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl):
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
In this reaction, silver (Ag⁺) and sodium (Na⁺) ions exchange places, forming silver chloride (AgCl), a white precipitate, and sodium nitrate (NaNO₃), which remains dissolved in solution. The (aq) indicates an aqueous solution, while (s) indicates a solid precipitate. This reaction is a classic example of a double replacement reaction driven by precipitate formation.
2. Gas Formation Reaction:
The reaction between hydrochloric acid (HCl) and sodium sulfide (Na₂S) produces hydrogen sulfide gas:
2HCl(aq) + Na₂S(aq) → H₂S(g) + 2NaCl(aq)
Here, hydrogen (H⁺) ions from HCl replace sodium (Na⁺) ions in Na₂S, forming hydrogen sulfide gas (H₂S), which is released as bubbles. The (g) indicates a gas.
3. Neutralization Reaction (Acid-Base Reaction):
A neutralization reaction is a special case of a double replacement reaction where an acid reacts with a base to produce water and a salt:
HCl(aq) + NaOH(aq) → H₂O(l) + NaCl(aq)
In this reaction, hydrogen ions (H⁺) from the acid (HCl) combine with hydroxide ions (OH⁻) from the base (NaOH) to form water (H₂O). The remaining ions form a salt (NaCl). The (l) denotes a liquid.
4. More Complex Examples:
Double replacement reactions can also involve more complex ions:
BaCl₂(aq) + (NH₄)₂SO₄(aq) → BaSO₄(s) + 2NH₄Cl(aq)
In this reaction, barium chloride reacts with ammonium sulfate to produce barium sulfate, a precipitate, and ammonium chloride.
Predicting Products in Double Replacement Reactions
To predict the products of a double replacement reaction, it's essential to understand the charges of the ions involved and their relative solubilities. Solubility rules are a set of guidelines that predict whether an ionic compound will dissolve in water. If a precipitate is predicted to form, the reaction will likely proceed.
For example, using the solubility rules, we can predict that silver chloride (AgCl) is insoluble in water, explaining why it precipitates in the first example. Conversely, sodium nitrate (NaNO₃) is soluble, remaining dissolved in the solution.
Conditions Favoring Double Replacement Reactions
Several factors can influence the occurrence and extent of a double replacement reaction:
- Solubility: As mentioned, the formation of an insoluble precipitate is a strong driving force.
- Gas Formation: The evolution of a gas from the solution also favors the reaction.
- Water Formation: The formation of water is highly favorable, as it's a stable and less reactive molecule.
- Concentration: Higher concentrations of reactants generally increase the reaction rate.
- Temperature: Increasing temperature often increases the reaction rate, but the effect can vary depending on the specific reaction.
Applications of Double Replacement Reactions
Double replacement reactions have numerous applications in various fields:
- Water Treatment: Reactions involving precipitation are used to remove unwanted ions from water.
- Chemical Synthesis: These reactions are fundamental in the synthesis of numerous inorganic and organic compounds.
- Analytical Chemistry: Precipitation reactions are used in qualitative analysis to identify the presence of specific ions.
- Metallurgy: Double replacement reactions can be used in metal extraction and purification processes.
- Pharmaceutical Industry: Many pharmaceutical compounds are synthesized through double replacement reactions.
Distinguishing Double Replacement from Other Reaction Types
It's crucial to distinguish double replacement reactions from other reaction types:
- Single Replacement Reactions: These involve one element replacing another in a compound. The general form is A + BX → AX + B.
- Synthesis Reactions (Combination Reactions): Two or more reactants combine to form a single product. The general form is A + B → AB.
- Decomposition Reactions: A single reactant breaks down into two or more products. The general form is AB → A + B.
- Combustion Reactions: A substance reacts rapidly with oxygen, producing heat and light.
Understanding the distinct features of each reaction type is key to accurate identification and prediction.
Conclusion
Double replacement reactions are a fundamental class of chemical reactions characterized by the exchange of ions between two ionic compounds. The formation of a precipitate, gas, or water often drives these reactions. By understanding the characteristics, predicting products using solubility rules, and recognizing the various applications, we gain a deeper appreciation for the significance of double replacement reactions in chemistry and its related fields. Accurate identification of these reactions relies on careful observation and a thorough understanding of chemical principles. The examples provided serve as a foundation for further exploration and a deeper understanding of the rich and diverse world of chemical reactions. Practicing with different examples and applying the concepts discussed will solidify your understanding and ability to identify these important reactions.
Latest Posts
Latest Posts
-
How Many Ounces Is 1 Cup Of Sour Cream
Mar 29, 2025
-
How Much Feet Is 48 Inches
Mar 29, 2025
-
Are Alternate Interior Angles Always Congruent
Mar 29, 2025
-
What Percent Of 40 Is 38
Mar 29, 2025
-
10 Is What Percentage Of 30
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Chemical Equation Represents A Double Replacement Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
