Which Equation Represents A Double Replacement Reaction
Kalali
Mar 31, 2025 · 6 min read
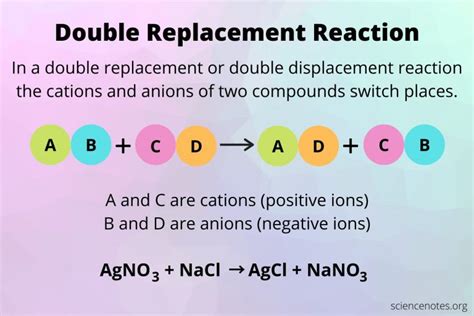
Table of Contents
Which Equation Represents a Double Replacement Reaction? A Comprehensive Guide
Double replacement reactions, also known as metathesis reactions, are a fundamental type of chemical reaction where two compounds exchange ions or elements to form two new compounds. Understanding how to identify these reactions, represented by their chemical equations, is crucial in chemistry. This comprehensive guide will delve deep into the characteristics of double replacement reactions, explore various examples, and equip you with the knowledge to confidently identify which equation represents this specific type of reaction.
Understanding Double Replacement Reactions
At the heart of a double replacement reaction lies the exchange of cations (positively charged ions) and anions (negatively charged ions) between two ionic compounds. This exchange happens in an aqueous solution (dissolved in water), where the ions are free to move and interact. The general form of a double replacement reaction can be represented as:
AB + CD → AD + CB
Where:
- A and C represent cations.
- B and D represent anions.
Key Characteristics of Double Replacement Reactions:
- Two reactants and two products: This is a defining feature. You'll always see two ionic compounds reacting to produce two new ionic compounds.
- Ions exchange partners: The cations and anions literally "swap places" forming new ionic bonds.
- Often occur in aqueous solutions: The reactants are usually dissolved in water, allowing the ions to be mobile and readily react.
- May lead to a precipitate, gas formation, or water formation: These are common indicators that a double replacement reaction has occurred. The reaction doesn't always result in all three; often, one is sufficient to signify the reaction.
Identifying Double Replacement Reactions: A Step-by-Step Approach
- Check for Aqueous Reactants: Ensure both reactants are dissolved in water (indicated by (aq) after the chemical formula).
- Identify the Ions: Break down each reactant into its constituent cations and anions.
- Predict the Products: Mentally swap the cations and anions.
- Check for Solubility: Consult a solubility chart to determine if any of the products are insoluble (will precipitate out of solution, indicated by (s)). This is a strong indicator of a double replacement reaction.
- Look for Gas Formation: Certain anion combinations produce gases (like CO₂ or H₂S), which will bubble out of the solution.
- Check for Water Formation: Reactions between acids and bases (neutralization reactions) are a subset of double replacement reactions that always produce water (H₂O).
Examples of Equations Representing Double Replacement Reactions
Let's examine several examples, highlighting the key features that identify them as double replacement reactions.
Example 1: Precipitation Reaction
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
- Reactants: Silver nitrate (AgNO₃) and sodium chloride (NaCl), both dissolved in water.
- Products: Silver chloride (AgCl), a white precipitate (insoluble solid), and sodium nitrate (NaNO₃), which remains dissolved.
- Ion Exchange: Silver (Ag⁺) ion from AgNO₃ combines with chloride (Cl⁻) ion from NaCl to form AgCl. Sodium (Na⁺) ion from NaCl combines with nitrate (NO₃⁻) ion from AgNO₃ to form NaNO₃.
This reaction is a classic example of a double replacement reaction where the formation of a precipitate (AgCl) clearly indicates the reaction has occurred.
Example 2: Gas Formation
Na₂S(aq) + 2HCl(aq) → 2NaCl(aq) + H₂S(g)
- Reactants: Sodium sulfide (Na₂S) and hydrochloric acid (HCl), both dissolved in water.
- Products: Sodium chloride (NaCl), which remains dissolved, and hydrogen sulfide (H₂S), a foul-smelling gas.
- Ion Exchange: Sodium (Na⁺) ions swap with hydrogen (H⁺) ions, and sulfide (S²⁻) ions swap with chloride (Cl⁻) ions.
The formation of the gaseous hydrogen sulfide is a strong indicator of a double replacement reaction.
Example 3: Neutralization Reaction
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
- Reactants: Sodium hydroxide (NaOH), a strong base, and hydrochloric acid (HCl), a strong acid, both dissolved in water.
- Products: Sodium chloride (NaCl), a salt, and water (H₂O).
- Ion Exchange: Sodium (Na⁺) ions from the base swap with hydrogen (H⁺) ions from the acid, forming water.
This is a neutralization reaction, a specific type of double replacement reaction characterized by the production of water and a salt. The heat released during this reaction is another key characteristic.
Example 4: A Reaction that Doesn't Fit
2Na(s) + Cl₂(g) → 2NaCl(s)
This equation does not represent a double replacement reaction. It's a synthesis reaction, where sodium (Na) and chlorine (Cl₂) combine directly to form sodium chloride (NaCl). There's no ion exchange involved. The reactants are not ionic compounds in aqueous solution.
Example 5: Another Non-Example
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
This is a combustion reaction, involving the burning of methane (CH₄) in oxygen (O₂). This is not a double replacement reaction as it's not an exchange of ions. It's a redox reaction (reduction-oxidation).
Common Misconceptions about Double Replacement Reactions
- All reactions producing precipitates are double replacement: While many double replacement reactions produce precipitates, not all reactions that produce a precipitate are double replacement. Some single replacement reactions also lead to precipitate formation.
- All reactions in aqueous solutions are double replacement: Many reactions occur in aqueous solutions that are not double replacement, including single replacement, decomposition, and synthesis reactions.
- All neutralization reactions are double replacement: This is true; acid-base neutralization reactions are a subset of double replacement reactions.
Advanced Considerations: Net Ionic Equations
Once you've identified a double replacement reaction, you can simplify the equation further using a net ionic equation. This equation only includes the ions that are directly involved in the reaction, excluding spectator ions (ions that don't change during the reaction).
For example, in the precipitation reaction of silver nitrate and sodium chloride:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
The net ionic equation is:
Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
The sodium (Na⁺) and nitrate (NO₃⁻) ions are spectator ions and are omitted from the net ionic equation.
Conclusion: Mastering Double Replacement Reactions
Identifying which equation represents a double replacement reaction requires a clear understanding of its defining characteristics: the exchange of ions between two ionic compounds in aqueous solution, often resulting in a precipitate, gas, or water formation. By systematically examining the reactants and products, identifying the ions, predicting the products, and checking for solubility or gas formation, you can confidently determine whether a given equation represents a double replacement reaction. Remember to utilize solubility charts and consider the possibility of net ionic equations for a more complete understanding. Practice with a wide array of examples is key to mastering this essential concept in chemistry. The examples provided above, along with the step-by-step approach and discussion of common misconceptions, should provide a solid foundation for identifying and understanding double replacement reactions. Continue exploring more complex examples to solidify your understanding and broaden your chemical knowledge.
Latest Posts
Latest Posts
-
21 Cm Is How Many Inches
Apr 01, 2025
-
What Is 5 4 As A Percentage
Apr 01, 2025
-
How Many Feet In 70 Inches
Apr 01, 2025
-
What Is Standard Form Of A Polynomial
Apr 01, 2025
-
El 5 Por Ciento De 1000
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Equation Represents A Double Replacement Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
