Which Pair Of Elements Would Form An Ionic Bond
Kalali
Mar 23, 2025 · 5 min read
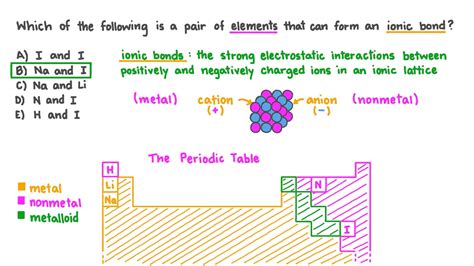
Table of Contents
Which Pair of Elements Would Form an Ionic Bond? A Deep Dive into Electrostatic Attraction
Ionic bonds, the fundamental forces holding together many of the compounds we encounter daily, arise from the powerful electrostatic attraction between oppositely charged ions. Understanding which pairs of elements readily form these bonds is crucial to grasping the principles of chemistry and predicting the properties of various materials. This comprehensive guide delves into the intricacies of ionic bonding, exploring the factors that determine its formation and providing numerous examples to solidify your understanding.
The Foundation of Ionic Bonding: Electronegativity Differences
The driving force behind ionic bond formation is the significant difference in electronegativity between two atoms. Electronegativity, a fundamental property of an element, quantifies its ability to attract electrons towards itself within a chemical bond. A large electronegativity difference signifies that one atom has a much stronger pull on the shared electrons than the other. This disparity leads to the transfer of electrons, not just sharing as seen in covalent bonds.
Understanding the Electronegativity Scale
Elements are arranged on the Pauling electronegativity scale, ranging from approximately 0.7 (for the most electropositive elements like cesium) to 4.0 (for the most electronegative element, fluorine). The greater the difference between the electronegativities of two atoms, the more likely they are to form an ionic bond. A general rule of thumb is that a difference of 1.7 or greater indicates a predominantly ionic bond. However, it's important to note that this is a guideline, and the actual character of the bond can vary depending on other factors such as the size of the ions and the surrounding environment.
Identifying Potential Ionic Bond Pairs: Metals and Nonmetals
The most reliable way to predict the formation of an ionic bond is by considering the positions of the elements on the periodic table. Metals, generally located on the left side of the table, tend to have low electronegativities and readily lose electrons to achieve a stable electron configuration (often an octet). Conversely, nonmetals, positioned on the right side, exhibit high electronegativities and readily gain electrons to achieve a stable configuration. The combination of a metal and a nonmetal creates the ideal scenario for electron transfer and the formation of an ionic bond.
Examples of Metal-Nonmetal Pairs Forming Ionic Bonds:
-
Sodium Chloride (NaCl): Sodium (Na), an alkali metal, readily loses one electron to achieve a stable electron configuration. Chlorine (Cl), a halogen, readily gains one electron to achieve a stable octet. The resulting positively charged sodium ion (Na⁺) and negatively charged chloride ion (Cl⁻) are strongly attracted to each other through electrostatic forces, forming the ionic compound sodium chloride (common table salt).
-
Magnesium Oxide (MgO): Magnesium (Mg), an alkaline earth metal, loses two electrons to become Mg²⁺. Oxygen (O), a highly electronegative nonmetal, gains two electrons to become O²⁻. The strong electrostatic attraction between the divalent ions forms the ionic compound magnesium oxide.
-
Potassium Iodide (KI): Potassium (K), an alkali metal, loses one electron to form K⁺. Iodine (I), a halogen, gains one electron to form I⁻. This results in the ionic compound potassium iodide.
-
Calcium Fluoride (CaF₂): Calcium (Ca), an alkaline earth metal, loses two electrons to form Ca²⁺. Fluorine (F), the most electronegative element, gains one electron each to form two F⁻ ions. The resulting compound, calcium fluoride, is held together by strong ionic bonds.
-
Aluminum Oxide (Al₂O₃): Aluminum (Al), a post-transition metal, loses three electrons to form Al³⁺. Oxygen (O) gains two electrons to form O²⁻. The ratio of aluminum to oxygen ions must balance the charges, resulting in the formula Al₂O₃.
Beyond the Simple Metal-Nonmetal Rule: Exceptions and Nuances
While the metal-nonmetal rule provides a valuable framework, it's crucial to acknowledge exceptions and nuances:
Polyatomic Ions:
Ionic compounds can also involve polyatomic ions, which are groups of atoms that carry a net charge. For example, sodium nitrate (NaNO₃) contains the sodium cation (Na⁺) and the nitrate anion (NO₃⁻). The ionic bond exists between the sodium ion and the nitrate polyatomic ion. Understanding the charges of polyatomic ions is crucial for predicting the formulas of ionic compounds.
Covalent Character in Ionic Compounds:
Even in predominantly ionic compounds, there is always a degree of covalent character. This is because the electrons are not completely transferred; there's always some degree of electron sharing. The extent of covalent character depends on the electronegativity difference and the size of the ions. Smaller, highly charged ions tend to exhibit greater covalent character due to increased polarisation of the electron cloud.
Lattice Energy: The Strength of Ionic Bonds:
The strength of an ionic bond is directly related to the lattice energy, which is the energy released when gaseous ions come together to form a solid crystal lattice. Several factors influence lattice energy, including the charges of the ions and the distance between them. Higher charges and smaller ionic radii lead to stronger electrostatic attractions and greater lattice energy.
Predicting Ionic Bond Formation: A Practical Approach
To predict whether a pair of elements will form an ionic bond, follow these steps:
-
Identify the elements: Determine the elements involved in the potential bond.
-
Locate them on the periodic table: Observe their position; metals are generally on the left, and nonmetals on the right.
-
Consider electronegativity differences: Consult an electronegativity table to determine the difference between the electronegativities of the two elements. A difference of 1.7 or greater suggests a predominantly ionic bond.
-
Determine ionic charges: Predict the likely charges of the ions based on their position on the periodic table and their valence electron configuration.
-
Balance charges: Ensure that the charges of the cations and anions balance out in the resulting formula.
Conclusion: Understanding the Power of Ionic Bonds
Ionic bonds are fundamental to the structure and properties of a vast range of materials. By understanding the underlying principles of electronegativity differences, metal-nonmetal interactions, and the influence of factors like lattice energy, we can confidently predict which pairs of elements are likely to form these strong electrostatic interactions. This knowledge is crucial for advancing our understanding of chemical reactions, material science, and countless other scientific disciplines. The examples provided throughout this guide offer a solid foundation for tackling more complex scenarios and further exploring the fascinating world of chemical bonding. Remember to always consider the exceptions and nuances to refine your predictions.
Latest Posts
Latest Posts
-
What Is The Boiling Point Of Water In Kelvin
Mar 24, 2025
-
30 Is What Percent Of 300
Mar 24, 2025
-
How Many Ml In 20 Ounces
Mar 24, 2025
-
What Is The Least Common Multiple Of 4 And 9
Mar 24, 2025
-
23 Inches Is How Many Feet
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Which Pair Of Elements Would Form An Ionic Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
