Which Properties Do Metalloids Share With Metals
Kalali
Mar 28, 2025 · 5 min read
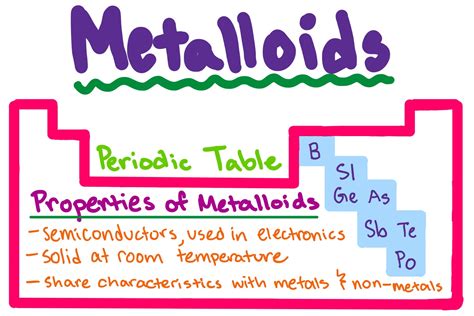
Table of Contents
Which Properties Do Metalloids Share with Metals?
Metalloids, also known as semimetals, occupy a fascinating middle ground in the periodic table, exhibiting properties that blend characteristics of both metals and nonmetals. Understanding their unique nature requires a closer examination of the specific properties they share with metals, and how these shared traits contribute to their diverse applications. This article delves deep into the similarities between metalloids and metals, exploring their physical and chemical characteristics.
Shared Physical Properties: A Blurred Line
While metalloids are distinctly different from metals in many ways, several key physical properties bridge the gap. These shared attributes often contribute to metalloids' usefulness in various technological applications.
1. Metallic Luster: A Shimmering Similarity
Many metalloids exhibit a metallic luster, although often less pronounced than in true metals. This shiny appearance arises from the interaction of light with their electrons. While not as bright or reflective as metals like silver or gold, metalloids like silicon and arsenic possess a characteristic sheen under specific lighting conditions. This shared characteristic highlights their intermediate nature between metals and nonmetals.
2. Electrical Conductivity: A Partial Embrace
A crucial property connecting metalloids and metals is electrical conductivity. However, unlike metals which are excellent conductors, metalloids demonstrate semiconductivity. This means their ability to conduct electricity is highly dependent on factors like temperature and the presence of impurities. At low temperatures, metalloids are poor conductors, but as temperature increases, their conductivity improves significantly. This unique behavior forms the basis of their use in semiconductor devices. This semi-conductivity is a key distinguishing feature from metals, though the underlying principle of electron movement is shared.
3. Thermal Conductivity: Heat Transfer, But Differently
Similar to electrical conductivity, metalloids also share the property of thermal conductivity with metals. They can transfer heat, albeit at a much lower rate compared to most metals. This thermal conductivity, again, is temperature-dependent and varies significantly among different metalloids. This moderate thermal conductivity is useful in applications where controlled heat transfer is required.
4. Malleability and Ductility: A Limited Similarity
While metals are generally known for their malleability (ability to be hammered into sheets) and ductility (ability to be drawn into wires), metalloids exhibit these properties to a much lesser extent. Some metalloids, like arsenic, are brittle and shatter easily under stress. Others, like silicon, show limited malleability and ductility at high temperatures, indicating a partial similarity but with significant limitations. This restricted malleability and ductility emphasizes their intermediate nature compared to the highly malleable and ductile properties seen in most metals.
5. Appearance and Density: Variable Similarities
Metalloids often display a metallic appearance in their crystalline form, although their color can vary significantly. This is a superficial similarity and does not reflect their underlying electronic structure. Furthermore, the density of metalloids falls within a range that overlaps with the density range of some metals, although significant variations exist within both groups.
Shared Chemical Properties: A Closer Look
The chemical properties of metalloids demonstrate a more complex overlap with metals, often displaying intermediate reactivity and oxidation states.
1. Formation of Alloys: A Shared Chemical Tendency
Metalloids readily form alloys with metals. These alloys often exhibit improved properties compared to the constituent metals. For instance, the addition of silicon to aluminum creates alloys with enhanced strength and casting properties. This capacity to form alloys highlights a chemical affinity between metalloids and metals. The ability to form stable metallic bonds, while not as strong as with metals alone, contributes to the utility of metalloid-metal alloys.
2. Oxidation States: A Variable Range
Metalloids exhibit multiple oxidation states, similar to transition metals. This allows them to participate in a wider range of chemical reactions. This ability to adopt varying oxidation states is a key factor in their versatility and makes them suitable for use as catalysts and dopants in various materials. However, the range of oxidation states is usually smaller than in many transition metals.
3. Reactivity with Acids and Bases: A Variable Response
The reactivity of metalloids with acids and bases is highly variable and dependent on the specific metalloid and the conditions of the reaction. Some metalloids can react with both acids and bases, while others are relatively inert. This varied reactivity contrasts with the generally consistent reactivity seen in many metals. The differing reactivity among metalloids emphasizes their transitional nature in the periodic table.
4. Ability to Form Covalent Bonds: A Shared Mechanism
Metalloids readily form covalent bonds, just like nonmetals. However, they also exhibit the ability to form metallic bonds, albeit weaker than those seen in pure metals. This dual ability to form both covalent and metallic bonds contributes to the unique structural properties and electrical behavior of metalloids. The coexistence of covalent and metallic bonding influences properties like the semiconductivity and variable reactivity discussed earlier.
5. Catalytic Properties: A Promising Overlap
Certain metalloids, particularly silicon and boron, exhibit catalytic properties in chemical reactions. This catalytic activity is a characteristic also shared with metals, although the mechanism and effectiveness may differ. The catalytic role of metalloids is an active area of research, with potential applications in various industrial processes.
Conclusion: The Intermediate Nature of Metalloids
The properties shared between metalloids and metals are a testament to the fluid nature of the periodic table. While metalloids exhibit many characteristics distinct from metals, a crucial set of shared physical and chemical properties makes them invaluable materials. Their semi-conductivity, ability to form alloys, variable oxidation states, and moderate reactivity with acids and bases contribute to their wide use in electronics, materials science, and various industrial processes.
The ongoing research into metalloids' behavior continues to uncover novel properties and applications. Their unique blend of metallic and nonmetallic characteristics makes them a fascinating and vital group of elements within the wider world of materials science. Their study serves as a powerful illustration of the importance of understanding the intricate relationship between chemical structure and physical properties. Their intermediate nature, rather than being a limitation, is the source of their unique value and enduring importance in modern technology and science.
Latest Posts
Latest Posts
-
What Is 24 25 As A Percent
Mar 31, 2025
-
What Tpye Of Reacgion Is Word Bank
Mar 31, 2025
-
80 Minutes Is How Many Hours
Mar 31, 2025
-
How Many Electrons Are In The Outer Shell Of Carbon
Mar 31, 2025
-
Cuanto Es 30 Grados Fahrenheit En Centigrados
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Properties Do Metalloids Share With Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
