Which Statement Best Describes The Law Of Conservation Of Energy
Kalali
Mar 13, 2025 · 6 min read
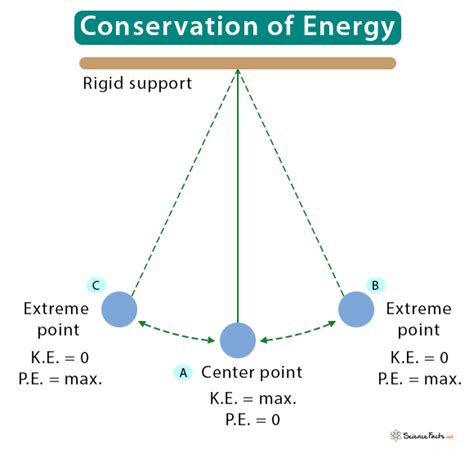
Table of Contents
Which Statement Best Describes the Law of Conservation of Energy?
The law of conservation of energy is a fundamental principle in physics, stating that energy cannot be created or destroyed, only transformed from one form to another. Understanding this law requires delving into its various interpretations and implications, addressing common misconceptions, and exploring its applications across diverse scientific fields. This comprehensive guide will not only answer the question of which statement best describes the law, but also provide a detailed exploration of the concept, solidifying your understanding of this cornerstone of physics.
Understanding the Core Principle: Energy Transformation, Not Creation or Destruction
The most accurate statement describing the law of conservation of energy is: The total energy of an isolated system remains constant over time. This means that within a closed system, where no energy enters or leaves, the sum of all forms of energy—kinetic, potential, thermal, chemical, nuclear, etc.—remains unchanged. While the energy may change form, the total amount always stays the same.
This is crucially different from saying energy is indestructible. Energy isn't a physical substance that simply persists. Instead, think of it as an abstract quantity representing the capacity to do work or cause change. This capacity can manifest in many ways:
- Kinetic Energy: Energy of motion. A moving car, a flying bird, even the molecules vibrating within a substance all possess kinetic energy.
- Potential Energy: Stored energy due to position or configuration. A book held high above the ground has gravitational potential energy, while a stretched spring has elastic potential energy.
- Thermal Energy (Heat): Energy associated with the random motion of particles within a substance. A hot cup of coffee has higher thermal energy than a cold one.
- Chemical Energy: Energy stored in the bonds between atoms and molecules. Burning wood releases chemical energy as heat and light.
- Nuclear Energy: Energy stored within the nucleus of an atom. Nuclear fission and fusion processes release enormous amounts of nuclear energy.
- Radiant Energy (Light): Energy carried by electromagnetic waves, such as light, radio waves, and X-rays.
The law of conservation of energy dictates that the sum of all these forms of energy within a closed system remains constant. If the kinetic energy of an object increases, it must be at the expense of another form of energy, such as potential energy or chemical energy. For example, a falling object loses potential energy and gains kinetic energy.
Addressing Common Misconceptions
Several misconceptions surround the law of conservation of energy. Let's address some common misunderstandings:
Misconception 1: Energy can be destroyed.
This is incorrect. While energy can be transferred or transformed, it cannot be destroyed. Even in processes that seem to "lose" energy, such as friction, the energy is merely converted into other forms, often heat, which disperses into the surroundings.
Misconception 2: The law applies only to isolated systems.
While the clearest and simplest application is to isolated systems, the law is more broadly applicable. Even in open systems (where energy exchange with the surroundings occurs), the total energy of the universe remains constant. This is a powerful statement, implying that energy is neither created nor destroyed on a universal scale. The accounting becomes more complex, but the underlying principle remains.
Misconception 3: Efficiency implies energy loss.
High efficiency means a large proportion of the initial energy is converted into the desired form. Inefficient processes simply convert more energy into less useful forms like heat, but the total energy remains constant. A lightbulb might only convert a small percentage of electrical energy into light; the rest becomes heat. The total energy remains the same; it's just not all in the desired form.
Applications of the Law of Conservation of Energy
The law of conservation of energy has far-reaching implications and is a fundamental principle in various scientific fields:
1. Engineering and Technology
Engineers rely on the law of conservation of energy to design and optimize systems. For example, in designing power plants, understanding energy conversion efficiency is critical for maximizing output and minimizing waste. The principle also governs the design of engines, motors, and other mechanical systems, ensuring energy is used effectively.
2. Thermodynamics
Thermodynamics extensively utilizes the law of conservation of energy. The first law of thermodynamics, in fact, is a statement of the law of conservation of energy applied to thermodynamic systems. It states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system. This principle is essential in understanding and predicting the behavior of thermal systems.
3. Environmental Science
Understanding energy flow in ecosystems is critical in environmental studies. The law of conservation of energy shows how energy is transferred through trophic levels in a food chain, from producers to consumers to decomposers. This understanding helps analyze ecological dynamics and predict environmental impacts.
4. Astrophysics and Cosmology
The law of conservation of energy is crucial in astrophysics and cosmology. It's used to model stellar evolution, the formation of galaxies, and the evolution of the universe itself. Understanding energy conversion processes in stars, such as nuclear fusion, relies heavily on this fundamental law.
5. Chemistry
Chemical reactions involve energy transformations. The law of conservation of energy is applied to determine enthalpy changes, reaction kinetics, and equilibrium constants. It is fundamental to understanding the energy released or absorbed during chemical processes.
Refining the Statement: Considering Mass-Energy Equivalence
While the statement "the total energy of an isolated system remains constant" is a good starting point, it becomes even more precise when we consider Einstein's famous equation, E=mc². This equation reveals the equivalence of mass and energy, demonstrating that mass itself is a form of energy. Therefore, a more comprehensive statement reflecting this relationship would be: In a closed system, the total energy, including the energy equivalent of mass, remains constant over time.
This refined statement acknowledges that mass can be converted into energy, and vice versa, as seen in nuclear reactions. In such reactions, a small amount of mass is converted into a significant amount of energy, consistent with the overall conservation of the combined energy-mass system. This demonstrates the profound connection between mass and energy, extending the law of conservation of energy to encompass both quantities.
Conclusion: A Cornerstone of Physics
The law of conservation of energy is one of the most fundamental and widely applicable principles in physics. While the simplest statement emphasizes the constancy of energy within a closed system, a more precise formulation integrates Einstein's mass-energy equivalence. Understanding this law and its implications is essential for comprehending numerous phenomena across various scientific disciplines, from the microscopic world of atoms and molecules to the vast expanse of the cosmos. Its enduring power and far-reaching applications solidify its position as a cornerstone of modern physics and a vital concept for anyone seeking a deeper understanding of the universe. This principle underpins technological advancements, scientific modeling, and our comprehension of the natural world. Its continued study and exploration promise to unveil even more profound insights into the workings of the universe.
Latest Posts
Latest Posts
-
How Many Cups Are In A Pound Of Rice
Jul 10, 2025
-
How Many Pounds Is One Ton Equal
Jul 10, 2025
-
A Warehouse Received 250 Orders In April
Jul 10, 2025
-
How Many Square Inches Are In 1 Square Foot
Jul 10, 2025
-
How Many Square Feet Is 3 4 Acre
Jul 10, 2025
Related Post
Thank you for visiting our website which covers about Which Statement Best Describes The Law Of Conservation Of Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.