Which Subatomic Particle Is Responsible For Chemical Bonding
Kalali
Mar 27, 2025 · 6 min read
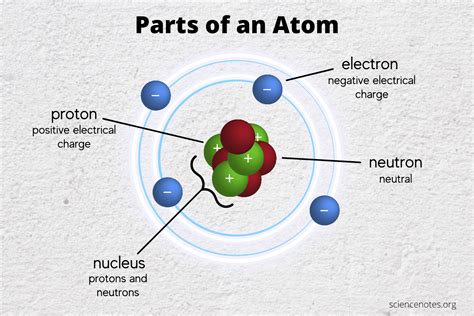
Table of Contents
Which Subatomic Particle is Responsible for Chemical Bonding?
Chemical bonding, the force that holds atoms together to form molecules and compounds, is a fundamental concept in chemistry. Understanding the nature of chemical bonds is crucial for comprehending the properties of matter and the behavior of chemical reactions. But which subatomic particle is the primary player in this atomic interaction? The answer, unequivocally, is the electron. While protons and neutrons contribute to the overall structure and identity of an atom, it's the electrons that orchestrate the formation of chemical bonds.
The Role of Electrons in Chemical Bonding
Electrons occupy specific energy levels, or shells, surrounding the nucleus of an atom. These shells have a limited capacity for electrons. The outermost shell, known as the valence shell, contains the valence electrons, which are directly involved in chemical bonding. The behavior of these valence electrons dictates how an atom will interact with other atoms.
Atoms strive to achieve a stable electron configuration, typically a full valence shell. This drive for stability is the fundamental driving force behind chemical bonding. There are several ways atoms can achieve this stability:
1. Ionic Bonding: The Transfer of Electrons
Ionic bonding involves the transfer of electrons from one atom to another. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond.
For example, consider the formation of sodium chloride (NaCl), common table salt. Sodium (Na) has one valence electron, while chlorine (Cl) has seven. Sodium readily loses its valence electron to achieve a stable electron configuration (like neon), becoming a Na⁺ cation. Chlorine gains this electron to complete its valence shell (like argon), becoming a Cl⁻ anion. The strong electrostatic attraction between the positively charged sodium ion and the negatively charged chloride ion constitutes the ionic bond holding the compound together.
Key characteristics of ionic bonding:
- High melting and boiling points: Due to the strong electrostatic forces between ions.
- Brittle nature: Disruption of the ion lattice structure leads to repulsion between like charges.
- Conductivity in molten or aqueous state: Free ions are capable of carrying electric current.
2. Covalent Bonding: The Sharing of Electrons
Covalent bonding involves the sharing of electrons between atoms. This sharing allows both atoms to achieve a stable electron configuration by filling their valence shells. The shared electrons are considered to belong to both atoms simultaneously.
Consider the formation of a water molecule (H₂O). Oxygen (O) has six valence electrons and needs two more to achieve a stable octet. Each hydrogen (H) atom has one valence electron and needs one more to fill its valence shell. Oxygen shares one electron with each hydrogen atom, and each hydrogen atom shares its electron with the oxygen atom. This sharing results in two covalent bonds, creating a water molecule.
Key characteristics of covalent bonding:
- Lower melting and boiling points compared to ionic compounds: Covalent bonds are generally weaker than ionic bonds.
- Often exist as gases, liquids, or low-melting solids: Reflecting the weaker intermolecular forces.
- Poor electrical conductivity: Electrons are localized in the bonds and are not free to move.
3. Metallic Bonding: A Sea of Electrons
Metallic bonding occurs in metals and is characterized by a "sea" of delocalized electrons. In metals, valence electrons are not associated with any particular atom but are free to move throughout the entire metal lattice. This mobility of electrons is responsible for many characteristic properties of metals.
Key characteristics of metallic bonding:
- High electrical and thermal conductivity: Free electrons can easily carry charge and energy.
- Malleability and ductility: The electron sea allows metal atoms to slide past each other without disrupting the structure.
- Lustrous appearance: Free electrons interact with light, causing reflection.
The insignificance of Protons and Neutrons in Chemical Bonding
While protons and neutrons reside in the nucleus and are crucial for defining the atomic number and mass number of an atom, they play a minimal direct role in chemical bonding. The strong nuclear force that holds protons and neutrons together within the nucleus is far too strong and short-ranged to directly influence the interactions between atoms involved in chemical bonding. Their primary influence is indirect, determining the number of protons, which in turn dictates the number of electrons and, consequently, the atom's bonding behavior.
The positive charge of the protons in the nucleus does, however, indirectly affect chemical bonding. It attracts the negatively charged electrons, holding them within the atom's electron cloud. The balance between the positive charge of the nucleus and the negative charge of the electrons determines the overall electronegativity of the atom, influencing the type and strength of bonds it forms.
Beyond Simple Bonds: Advanced Bonding Concepts
While the simple models of ionic, covalent, and metallic bonding provide a foundational understanding, the reality of chemical bonding is often more nuanced. Many molecules exhibit a mixture of bonding characteristics, falling somewhere between purely ionic, purely covalent, or purely metallic. This is often characterized by concepts such as:
-
Polar Covalent Bonds: These bonds occur when electrons are shared unequally between atoms with differing electronegativities. This creates a dipole moment, with one atom having a partial positive charge and the other having a partial negative charge. Water molecules are a classic example.
-
Coordinate Covalent Bonds (Dative Bonds): In this type of bond, both shared electrons originate from the same atom. This is common in complex ions and coordination compounds.
-
Hydrogen Bonding: A special type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule. Hydrogen bonding is crucial for the properties of water and many biological molecules.
-
Van der Waals Forces: Weak intermolecular forces that arise from temporary fluctuations in electron distribution. These forces are significant in determining the physical properties of nonpolar molecules.
Conclusion: Electrons are the Architects of Chemical Bonding
In conclusion, while the nucleus of an atom plays a fundamental role in its overall identity and stability, it is the electrons, specifically the valence electrons, that are unequivocally responsible for chemical bonding. Their behavior—transfer, sharing, or delocalization—dictates the formation of ionic, covalent, and metallic bonds, respectively. Understanding the role of electrons in chemical bonding is paramount to comprehending the structure, properties, and reactivity of matter in the world around us. The diverse ways electrons interact lay the foundation for the vast complexity of chemical systems, from simple molecules to intricate biological macromolecules. By understanding the electron's role, we unlock a fundamental understanding of the world at the atomic level.
Latest Posts
Latest Posts
-
Cuanto Es 52 Grados Fahrenheit En Centigrados
Mar 30, 2025
-
What Is 425 Degrees Fahrenheit In Celsius
Mar 30, 2025
-
Where Do Organisms Get The Energy They Need To Survive
Mar 30, 2025
-
What Element Has 6 Protons 7 Neutrons And 6 Electrons
Mar 30, 2025
-
How Long Is 120 Inches In Feet
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Which Subatomic Particle Is Responsible For Chemical Bonding . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
