Write A Balanced Equation For The Decomposition Of Hydrogen Peroxide
Kalali
Mar 31, 2025 · 5 min read
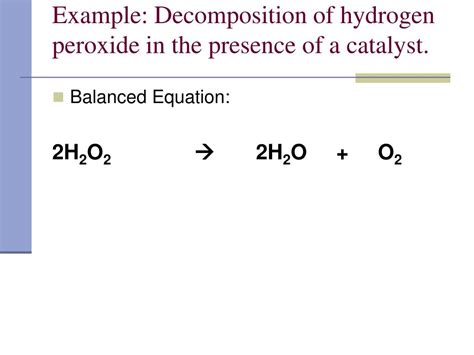
Table of Contents
The Balanced Equation for the Decomposition of Hydrogen Peroxide: A Deep Dive
Hydrogen peroxide (H₂O₂) is a common chemical compound with diverse applications, from antiseptic solutions to industrial bleaching agents. Understanding its decomposition is crucial in various fields, from chemistry education to industrial process optimization. This article delves into the balanced equation for the decomposition of hydrogen peroxide, exploring the different factors influencing the reaction rate and the various applications stemming from this fundamental chemical process.
The Basic Decomposition Reaction
The decomposition of hydrogen peroxide is a simple yet significant chemical reaction. In its most basic form, it's the breakdown of hydrogen peroxide into water (H₂O) and oxygen (O₂). This process can be represented by the following unbalanced equation:
H₂O₂ → H₂O + O₂
This equation is unbalanced because the number of oxygen atoms is not equal on both sides. To achieve a balanced equation, we need to ensure the number of each type of atom is the same on both the reactant (left-hand side) and product (right-hand side) sides of the equation.
Balancing the Equation: A Step-by-Step Guide
Balancing chemical equations involves adjusting the stoichiometric coefficients—the numbers placed in front of the chemical formulas—until the number of atoms of each element is equal on both sides. Here's how to balance the equation for the decomposition of hydrogen peroxide:
-
Identify the elements: We have hydrogen (H) and oxygen (O).
-
Count the atoms: On the left side (reactants), we have 2 hydrogen atoms and 2 oxygen atoms. On the right side (products), we have 2 hydrogen atoms and 2 oxygen atoms.
-
Balance the oxygen atoms: Note that the oxygen atoms are already balanced.
-
Balance the hydrogen atoms: The hydrogen atoms are also balanced.
Therefore, the balanced equation for the decomposition of hydrogen peroxide is:
2H₂O₂ → 2H₂O + O₂
This equation clearly shows that two molecules of hydrogen peroxide decompose to form two molecules of water and one molecule of oxygen gas. This balanced equation is essential for accurate stoichiometric calculations, allowing us to predict the amounts of reactants and products involved in the reaction.
Factors Affecting the Decomposition Rate
The rate at which hydrogen peroxide decomposes is influenced by several factors:
1. Temperature:
Increasing the temperature increases the kinetic energy of the hydrogen peroxide molecules. This leads to more frequent and energetic collisions, increasing the likelihood of successful collisions that break the O-O bond and initiate the decomposition process. Higher temperatures, therefore, accelerate the decomposition rate.
2. Catalysts:
Catalysts are substances that increase the rate of a chemical reaction without being consumed in the process. Several substances can catalyze the decomposition of hydrogen peroxide, including:
-
Manganese dioxide (MnO₂): This is a commonly used catalyst in demonstrations, visibly accelerating the decomposition and producing a rapid stream of oxygen gas.
-
Potassium iodide (KI): Another effective catalyst, KI speeds up the decomposition through a different reaction mechanism.
-
Enzymes (e.g., catalase): Biological systems utilize enzymes like catalase to catalyze the decomposition of hydrogen peroxide, protecting cells from the damaging effects of this reactive compound. Catalase is found in many living organisms, including humans.
The presence of a catalyst lowers the activation energy of the reaction, making it easier for the molecules to react and thus increasing the decomposition rate.
3. Concentration:
Higher concentrations of hydrogen peroxide lead to a faster decomposition rate. This is because a greater number of hydrogen peroxide molecules are available to react, increasing the frequency of successful collisions.
4. Surface Area:
In heterogeneous catalysis (where the catalyst is in a different phase than the reactant), increasing the surface area of the catalyst exposes more active sites for the reaction to occur, resulting in a faster decomposition rate. For example, finely powdered MnO₂ will decompose H₂O₂ much faster than a large lump of MnO₂.
5. Light:
Exposure to light, particularly ultraviolet (UV) light, can also accelerate the decomposition of hydrogen peroxide. UV light provides the energy needed to break the O-O bond, initiating the decomposition reaction.
Applications of Hydrogen Peroxide Decomposition
The decomposition of hydrogen peroxide, both catalyzed and uncatalyzed, finds applications in several areas:
1. Rocket Propulsion:
Concentrated hydrogen peroxide has been used as a monopropellant in rocket engines. Its decomposition, often catalyzed by metals such as silver or platinum, generates hot water vapor and oxygen gas, providing thrust.
2. Wastewater Treatment:
Hydrogen peroxide is used as an oxidizing agent in wastewater treatment to break down pollutants. Its decomposition generates reactive oxygen species that oxidize organic contaminants.
3. Disinfectant and Antiseptic:
The antiseptic properties of hydrogen peroxide are attributed to its oxidizing ability and the release of oxygen gas upon decomposition. This reactive oxygen damages the cell membranes and cellular components of microorganisms, leading to their inactivation.
4. Bleaching Agent:
Hydrogen peroxide's bleaching action relies on its oxidizing power. The release of oxygen during decomposition bleaches various substances by breaking down colored compounds.
5. Chemical Synthesis:
The decomposition reaction can be used as a source of oxygen in various chemical syntheses, providing a controlled and relatively clean way to introduce oxygen into a reaction.
6. Educational Demonstrations:
The decomposition of hydrogen peroxide catalyzed by manganese dioxide is a classic chemistry demonstration illustrating the concept of catalysis and the production of a gas. The rapid evolution of oxygen gas is visually striking.
Safety Considerations
While hydrogen peroxide is used in many applications, it's essential to handle it with caution. Concentrated hydrogen peroxide solutions are corrosive and can cause burns. Furthermore, the rapid decomposition of hydrogen peroxide can generate heat and pressure, particularly in confined spaces or when catalyzed, posing a safety risk. Always follow appropriate safety procedures and wear personal protective equipment when working with hydrogen peroxide.
Conclusion
The balanced equation for the decomposition of hydrogen peroxide (2H₂O₂ → 2H₂O + O₂) is a fundamental concept in chemistry. Understanding this reaction and the factors that influence its rate is crucial for applications ranging from rocket propulsion to antiseptic use. The reaction's versatility and the relative ease of controlling its rate make hydrogen peroxide a valuable compound with extensive applications in various scientific and industrial fields. Further research into more efficient catalysts and a deeper understanding of the reaction mechanisms will likely lead to even more innovative applications of this ubiquitous compound in the future. Remember always to prioritize safety when working with hydrogen peroxide and other chemicals.
Latest Posts
Latest Posts
-
What Is 5 4 As A Percentage
Apr 01, 2025
-
How Many Feet In 70 Inches
Apr 01, 2025
-
What Is Standard Form Of A Polynomial
Apr 01, 2025
-
El 5 Por Ciento De 1000
Apr 01, 2025
-
How Many Feet Is 110 In
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write A Balanced Equation For The Decomposition Of Hydrogen Peroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
