Does Co Have Dipole Dipole Forces
Kalali
Mar 30, 2025 · 6 min read
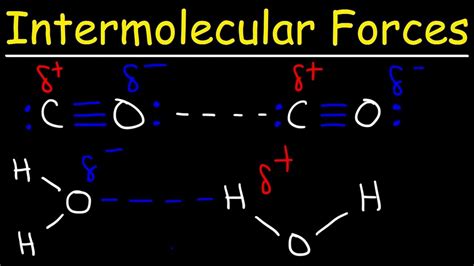
Table of Contents
Does CO Have Dipole-Dipole Forces? Understanding Intermolecular Forces in Carbon Monoxide
Carbon monoxide (CO), a simple yet fascinating molecule, often sparks discussions regarding its intermolecular forces. While its linear structure might initially suggest a nonpolar molecule devoid of dipole-dipole interactions, a closer examination reveals a more nuanced reality. This article delves into the intricacies of CO's molecular structure, its polarity, and the types of intermolecular forces it exhibits, clarifying the presence (or lack thereof) of dipole-dipole forces.
Understanding Intermolecular Forces
Before diving into the specifics of CO, let's establish a foundational understanding of intermolecular forces (IMFs). These forces are the attractions between molecules, significantly impacting a substance's physical properties like boiling point, melting point, and viscosity. The strength of IMFs varies greatly, influencing the state of matter at a given temperature and pressure.
The primary types of IMFs include:
-
London Dispersion Forces (LDFs): These are the weakest IMFs, present in all molecules, regardless of polarity. They arise from temporary, instantaneous dipoles created by the random movement of electrons. Larger, more polarizable molecules exhibit stronger LDFs.
-
Dipole-Dipole Forces: These forces occur between polar molecules, meaning molecules with a permanent dipole moment. The partially positive end of one molecule is attracted to the partially negative end of another. These forces are stronger than LDFs.
-
Hydrogen Bonding: This is a special type of dipole-dipole interaction involving a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). Hydrogen bonds are the strongest type of IMF.
-
Ion-Dipole Forces: These forces occur between ions and polar molecules. The charged ion is attracted to the oppositely charged end of the polar molecule.
The Molecular Structure and Polarity of CO
Carbon monoxide possesses a linear molecular geometry with a carbon atom double-bonded to an oxygen atom (C≡O). Oxygen is significantly more electronegative than carbon, meaning it attracts electrons more strongly. This electronegativity difference leads to a polar molecule with a dipole moment. The oxygen atom carries a partial negative charge (δ-), while the carbon atom carries a partial positive charge (δ+). This asymmetry in charge distribution is crucial in determining the types of IMFs present.
Visualizing the Dipole Moment
Imagine the CO molecule as a tiny bar magnet. The oxygen end acts like the south pole (δ-), and the carbon end acts like the north pole (δ+). This inherent polarity is responsible for its interactions with other CO molecules and other polar molecules.
Does CO Exhibit Dipole-Dipole Forces?
The answer is a resounding yes. Because carbon monoxide is a polar molecule with a permanent dipole moment, it exhibits dipole-dipole forces. The partially positive carbon atom of one CO molecule is attracted to the partially negative oxygen atom of another CO molecule. These attractions contribute significantly to CO's physical properties.
The Role of London Dispersion Forces in CO
While dipole-dipole forces are present and relatively strong in CO, it's important to acknowledge the contribution of London Dispersion Forces (LDFs). Even though CO is polar, LDFs are still present and contribute to the overall intermolecular attraction. The strength of LDFs in CO is less than the dipole-dipole forces, but they still play a role in determining its physical properties.
Comparing the Strength of IMFs in CO
The combination of dipole-dipole forces and London Dispersion Forces makes the intermolecular attractions in CO stronger than those found in nonpolar molecules of similar size, like N₂ or O₂. This explains why CO has a higher boiling point (-191.5 °C) compared to N₂ (-195.8 °C) and O₂ (-183.0 °C), despite having similar molecular weights.
The Significance of Dipole-Dipole Forces in CO's Properties
The presence of dipole-dipole forces profoundly influences CO's physical properties. This interaction contributes to:
-
Higher Boiling Point: Compared to nonpolar molecules of similar size, CO has a higher boiling point due to the stronger intermolecular attractions.
-
Solubility in Polar Solvents: CO is more soluble in polar solvents like water than in nonpolar solvents because of the dipole-dipole interactions between CO and the solvent molecules.
-
Liquid State at Lower Temperatures: The stronger intermolecular forces allow CO to exist in the liquid state at lower temperatures compared to nonpolar molecules.
Distinguishing Dipole-Dipole Forces from Other IMFs in CO
It's crucial to differentiate dipole-dipole forces from other IMFs present in CO. Although LDFs are also present, the dominant intermolecular force responsible for the properties of CO is the dipole-dipole interaction. Hydrogen bonding is not present because hydrogen is not bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine.
Conclusion: Dipole-Dipole Forces are Key to Understanding CO
Carbon monoxide's intermolecular forces are a captivating example of how molecular structure dictates physical properties. The presence of a dipole moment due to the difference in electronegativity between carbon and oxygen results in significant dipole-dipole interactions. While London Dispersion Forces are also present, the dipole-dipole forces are the dominant IMFs, influencing CO's boiling point, solubility, and other physical characteristics. Understanding these interactions provides a crucial framework for comprehending the behavior and properties of this important molecule.
Frequently Asked Questions (FAQs)
Q1: Is carbon monoxide a polar molecule?
A1: Yes, carbon monoxide is a polar molecule due to the difference in electronegativity between the carbon and oxygen atoms. Oxygen is more electronegative, resulting in a partial negative charge on the oxygen and a partial positive charge on the carbon.
Q2: What are the main intermolecular forces present in CO?
A2: The main intermolecular forces in CO are dipole-dipole forces and London Dispersion Forces (LDFs). Dipole-dipole forces are the dominant interaction due to CO's polarity.
Q3: Why does CO have a higher boiling point than N₂ and O₂?
A3: CO has a higher boiling point than N₂ and O₂ due to the presence of stronger dipole-dipole forces in addition to LDFs. N₂ and O₂ are nonpolar molecules and only exhibit LDFs.
Q4: Is hydrogen bonding present in CO?
A4: No, hydrogen bonding is not present in CO. Hydrogen bonding requires a hydrogen atom bonded to a highly electronegative atom (O, N, or F), which is not the case in CO.
Q5: How do dipole-dipole forces affect the solubility of CO?
A5: Dipole-dipole forces enhance the solubility of CO in polar solvents. The interaction between the dipole moment of CO and the dipole moments of the solvent molecules promotes dissolution.
This comprehensive exploration of intermolecular forces in carbon monoxide clarifies the presence of significant dipole-dipole interactions, which, combined with London Dispersion Forces, dictate its unique physical characteristics. Understanding these forces is essential for comprehending the behavior and applications of this important molecule across various scientific disciplines.
Latest Posts
Latest Posts
-
How Many Cups Is 2000 Ml
Apr 01, 2025
-
90 Out Of 125 As A Percentage
Apr 01, 2025
-
Keq Is Equal To Delta G
Apr 01, 2025
-
How Many Grams Is 400 Mg
Apr 01, 2025
-
How Many Feet Is 136 Inches
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Does Co Have Dipole Dipole Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
