How Are Elements And Compounds Similar
Kalali
Mar 12, 2025 · 6 min read
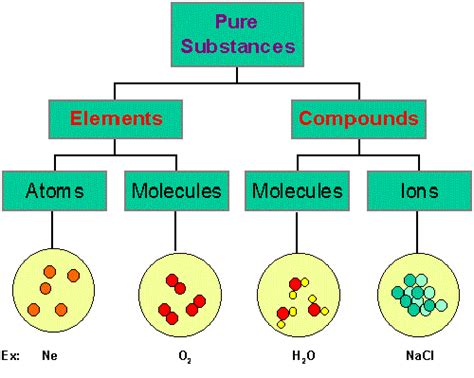
Table of Contents
How Are Elements and Compounds Similar? Exploring the Overlapping Properties of Matter
Understanding the fundamental building blocks of matter—elements and compounds—is crucial to grasping the complexities of chemistry. While they differ significantly in their composition and properties, there are surprising similarities between elements and compounds that often go unnoticed. This article delves deep into these similarities, exploring their shared characteristics and highlighting the subtle nuances that differentiate them. We will examine their shared states of matter, their ability to participate in chemical reactions, and their role in forming mixtures, providing a comprehensive understanding of their interconnectedness.
Shared Physical States: Solid, Liquid, and Gas
One striking similarity between elements and compounds lies in their ability to exist in all three fundamental states of matter: solid, liquid, and gas. This is independent of their chemical composition. For example, the element oxygen (O₂) can exist as a gas at room temperature, but can be liquefied and even solidified under appropriate conditions of temperature and pressure. Similarly, the compound water (H₂O) exists as a solid (ice), liquid (water), and gas (steam), depending on the temperature. This demonstrates that the physical state is a property not solely determined by chemical composition, but also by external factors like temperature and pressure. Both elements and compounds can undergo phase transitions—changes from one state to another—under varying conditions.
Transitions and Properties within States
Furthermore, the properties within each state are not solely dependent on whether the substance is an element or a compound. For example, the density of a solid element like iron can be compared to the density of a solid compound like quartz. While the chemical makeup is vastly different, both exhibit the characteristic rigidity and fixed shape associated with the solid state. Similarly, the viscosity (resistance to flow) can be compared between liquid elements like mercury and liquid compounds like ethanol. These comparisons reveal that physical properties within a particular state of matter are not exclusive to either elements or compounds.
Participation in Chemical Reactions: Reactivity and Stability
Both elements and compounds are capable of participating in chemical reactions, though their reactivity and stability often differ significantly. Elements, in their pure form, can react with other elements or compounds to form new substances. For example, sodium (Na), a highly reactive element, readily reacts with chlorine (Cl₂) to form the compound sodium chloride (NaCl), or table salt. Compounds, similarly, can undergo chemical reactions, either decomposing into simpler substances or reacting with other elements or compounds to create new compounds. For instance, heating calcium carbonate (CaCO₃) results in its decomposition into calcium oxide (CaO) and carbon dioxide (CO₂).
Reactivity and the Periodic Table
The reactivity of an element is often predictable based on its position in the periodic table. Elements in the same group (vertical column) often exhibit similar chemical behaviors due to their similar electronic configurations. However, the reactivity of compounds is significantly more complex and depends on several factors, including the types of elements present, the arrangement of atoms, and the presence of functional groups. This doesn't negate the fact that both elements and compounds can undergo chemical changes.
Formation of Mixtures: Homogeneous and Heterogeneous
Elements and compounds can both be components of mixtures. Mixtures are physical combinations of two or more substances, where the individual substances retain their chemical identities. Mixtures can be homogeneous, meaning the composition is uniform throughout (e.g., saltwater), or heterogeneous, meaning the composition is not uniform (e.g., sand and water). Pure elements can form mixtures with each other (e.g., bronze, an alloy of copper and tin), and pure compounds can form mixtures with each other (e.g., air, a mixture of various gases) or with elements (e.g., seawater, a mixture of water and dissolved salts).
Separating Mixture Components
The ability of both elements and compounds to be part of mixtures also leads to a similarity in separation techniques. Techniques such as filtration, distillation, and chromatography can be used to separate the components of mixtures regardless of whether those components are elements or compounds. The choice of separation technique depends on the properties of the mixture's components and their differences in physical properties like boiling point or solubility. This highlights another commonality: both elements and compounds can be separated from mixtures using appropriate physical methods.
Energy Changes in Reactions: Exothermic and Endothermic Processes
Both elements and compounds undergo chemical reactions that involve energy changes. These reactions can be exothermic, releasing energy in the form of heat or light, or endothermic, absorbing energy from their surroundings. For example, the combustion of methane (a compound) is an exothermic reaction, releasing a significant amount of heat. Conversely, the decomposition of water (a compound) into hydrogen and oxygen (elements) is an endothermic reaction, requiring energy input to proceed. Similarly, the reaction of sodium (an element) with water is exothermic, while the formation of some metal oxides from their elements can be endothermic.
Energy Levels and Bond Formation
The energy changes involved in reactions are related to the changes in the energy levels of the atoms and molecules involved. The formation of chemical bonds releases energy, while the breaking of chemical bonds requires energy input. Whether a reaction is exothermic or endothermic depends on the relative amounts of energy released and absorbed during bond breaking and bond formation. This is a fundamental principle applicable to both element- and compound-involving reactions.
Differences Despite Similarities: A Crucial Distinction
While the similarities discussed above underscore the interconnectedness of elements and compounds, it is crucial to emphasize their fundamental difference: composition. Elements are pure substances consisting of only one type of atom, while compounds are pure substances composed of two or more different types of atoms chemically bonded in fixed proportions. This compositional difference leads to significant variations in their chemical and physical properties. Elements cannot be broken down into simpler substances by chemical means, whereas compounds can be decomposed into their constituent elements through chemical reactions.
Properties Unique to Compounds
Compounds exhibit unique properties that are entirely different from the properties of their constituent elements. For example, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a harmless, crystalline solid with entirely different properties. This is a key differentiating factor between elements and compounds.
Conclusion: Interdependence in the Material World
In conclusion, while elements and compounds differ significantly in their composition—the fundamental building block of matter—they share many similarities in their physical behavior and their ability to participate in chemical reactions. They both exist in various states of matter, undergo phase transitions, participate in exothermic and endothermic reactions, and form part of mixtures. Understanding these similarities alongside their crucial differences is essential for comprehending the interconnectedness of matter and the fundamental principles of chemistry. The similarities highlight the underlying physical laws governing matter regardless of its complexity, while the differences underscore the power of chemical bonding in creating substances with unique and often unpredictable properties. This interwoven relationship between elements and compounds is a cornerstone of the material world we inhabit.
Latest Posts
Latest Posts
-
Is Magnesium A Metal Nonmetal Or Metalloid
Mar 12, 2025
-
What Is The Difference Between Reactants And Products
Mar 12, 2025
-
Is A Candy Bar Melting Convection
Mar 12, 2025
-
Common Multiples Of 5 And 7
Mar 12, 2025
-
86 Inches Is How Many Feet
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about How Are Elements And Compounds Similar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
