How Many Electrons Does Cl Have
Kalali
Mar 31, 2025 · 5 min read
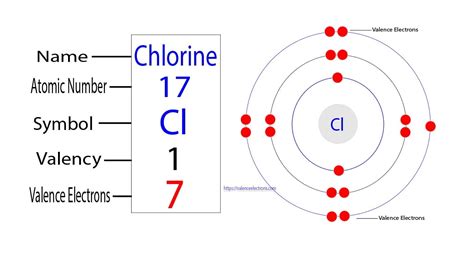
Table of Contents
How Many Electrons Does Chlorine (Cl) Have? A Deep Dive into Atomic Structure
Chlorine (Cl), a ubiquitous element found in everyday life from table salt to swimming pools, holds a fascinating place in the periodic table. Understanding its electron configuration is key to grasping its chemical behavior and reactivity. This in-depth article explores the number of electrons in a chlorine atom, delving into the fundamentals of atomic structure, electron shells, and valence electrons—critical concepts in chemistry and related fields.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we determine the number of electrons in chlorine, let's establish the basics of atomic structure. An atom is the fundamental building block of matter, composed of three subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons.
Chlorine's Atomic Number and Electron Configuration
Chlorine's atomic number is 17. This crucial number indicates that a neutral chlorine atom possesses 17 protons in its nucleus. Consequently, a neutral chlorine atom also contains 17 electrons.
The arrangement of these 17 electrons isn't haphazard; they occupy specific energy levels or electron shells surrounding the nucleus. This arrangement, known as the electron configuration, dictates the atom's chemical properties and reactivity. For chlorine, the electron configuration is:
1s² 2s² 2p⁶ 3s² 3p⁵
Let's break down this notation:
- 1s²: The first energy level (n=1) contains one subshell (s), holding a maximum of two electrons. Chlorine has two electrons in this shell.
- 2s²: The second energy level (n=2) also has an s subshell, which holds another two electrons.
- 2p⁶: The second energy level also contains three p subshells (px, py, pz), each capable of holding up to two electrons. In chlorine, all six positions in the 2p subshell are filled.
- 3s²: The third energy level (n=3) starts with an s subshell containing two electrons.
- 3p⁵: Finally, the third energy level's three p subshells hold five electrons. This incomplete outermost shell is crucial to chlorine's reactivity.
Valence Electrons: The Key to Chemical Bonding
The electrons in the outermost shell are called valence electrons. These electrons are involved in chemical bonding, determining how an atom interacts with other atoms to form molecules or compounds. In chlorine's case, the 3s² 3p⁵ configuration indicates it has 7 valence electrons. This is why chlorine is highly reactive; it readily gains one electron to achieve a stable octet (eight electrons) in its outermost shell, resembling the electron configuration of a noble gas.
Isotopes of Chlorine and Electron Number
While the number of protons defines an element, the number of neutrons can vary, creating isotopes. Chlorine has two naturally occurring isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). These isotopes differ in the number of neutrons but have the same number of electrons (17) in a neutral atom. The difference lies in their mass number (protons + neutrons).
- ³⁵Cl has 18 neutrons (17 protons + 18 neutrons = 35).
- ³⁷Cl has 20 neutrons (17 protons + 20 neutrons = 37).
The difference in neutron number affects the mass of the atom but doesn't alter the number of electrons in a neutral atom. The electron configuration remains the same for both isotopes.
Chlorine's Reactivity and Electron Gain
Chlorine's high reactivity stems directly from its seven valence electrons. To achieve a stable octet, it readily gains one electron, forming a negatively charged chloride ion (Cl⁻). This electron gain is a fundamental aspect of chlorine's chemistry, leading to the formation of numerous ionic compounds, such as sodium chloride (NaCl, table salt).
The strong electronegativity of chlorine, its tendency to attract electrons, further enhances its ability to form ionic bonds. This characteristic makes chlorine a powerful oxidizing agent, capable of accepting electrons from other atoms or molecules.
Applications of Understanding Chlorine's Electron Configuration
Understanding chlorine's electron configuration and its implications has far-reaching applications:
- Predicting Chemical Reactions: Knowing the number of valence electrons allows chemists to predict how chlorine will react with other elements or compounds.
- Designing New Materials: The understanding of atomic structure and electron interactions is crucial for designing novel materials with specific properties.
- Understanding Biological Processes: Chlorine plays essential roles in biological systems, and its interactions with other molecules are governed by its electron configuration.
- Environmental Chemistry: Chlorine's reactivity and its presence in various environmental contexts (e.g., chlorinated solvents, chlorofluorocarbons) are important considerations for environmental monitoring and remediation efforts.
Beyond the Basics: Quantum Mechanics and Electron Orbitals
While the simplified shell model provides a useful picture, a more accurate representation of electron distribution involves quantum mechanics and the concept of atomic orbitals. Each electron isn't confined to a specific orbit but occupies an atomic orbital, a region of space where there's a high probability of finding the electron. These orbitals are described by quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number). A deeper understanding of these concepts is necessary for advanced studies in chemistry and physics.
Conclusion: The Significance of 17 Electrons
In summary, a neutral chlorine atom possesses 17 electrons, arranged according to its electron configuration (1s² 2s² 2p⁶ 3s² 3p⁵). Its seven valence electrons are responsible for its high reactivity and its tendency to gain one electron, forming the stable chloride ion (Cl⁻). This fundamental understanding of chlorine's atomic structure is vital for comprehending its chemical behavior, its role in various chemical reactions, and its significance in numerous applications across various scientific disciplines. The number 17, therefore, is far more than just a simple numerical value; it's the key to unlocking a wealth of knowledge about this crucial and versatile element. From the formation of table salt to its crucial roles in industrial processes and biological systems, chlorine's 17 electrons are at the heart of its remarkable properties and widespread importance.
Latest Posts
Latest Posts
-
Number Of Energy Levels In Oxygen
Apr 01, 2025
-
What Is The Wavelength Of A 2 99 Hz Wave
Apr 01, 2025
-
36 Is 30 Percent Of What Number
Apr 01, 2025
-
How Many Hours Is 800 Minutes
Apr 01, 2025
-
13 Out Of 17 As A Percentage
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Cl Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
