Is Baking Soda A Compound Or Mixture
Kalali
Mar 17, 2025 · 6 min read
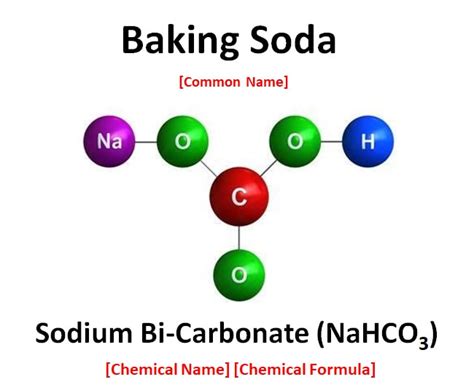
Table of Contents
Is Baking Soda a Compound or a Mixture? A Deep Dive into Chemical Composition
Baking soda, a staple in many kitchens, is more than just a leavening agent; it's a fascinating chemical substance that sparks curiosity about its fundamental nature. Is it a compound or a mixture? This seemingly simple question opens the door to a deeper understanding of chemistry and the distinctions between these two fundamental classifications of matter. Let's embark on an in-depth exploration to definitively answer this question and illuminate the science behind baking soda.
Understanding Compounds and Mixtures: A Foundational Overview
Before we dive into the specifics of baking soda, it's crucial to establish a clear understanding of the difference between compounds and mixtures. This distinction forms the bedrock of our investigation.
What is a Compound?
A compound is a pure substance formed when two or more different chemical elements are chemically bonded together. These bonds are strong, requiring significant energy to break. The resulting substance has entirely different properties than its constituent elements. For example, water (H₂O) is a compound composed of hydrogen and oxygen; it exhibits properties completely unlike those of either hydrogen gas or oxygen gas. Key characteristics of compounds include:
- Fixed ratio of elements: The elements within a compound are always present in a specific and consistent ratio, defined by its chemical formula.
- Uniform composition: A compound has a homogeneous composition throughout; every part of it is identical.
- Distinct properties: The properties of a compound are unique and different from the properties of its constituent elements.
- Chemical changes required for separation: Separating the elements of a compound requires chemical reactions, not simple physical methods.
What is a Mixture?
A mixture, unlike a compound, is a physical combination of two or more substances that are not chemically bonded. The individual substances retain their own chemical properties within the mixture. These substances can be easily separated using physical methods like filtration, distillation, or evaporation. Mixtures can be categorized into two types:
- Homogeneous mixtures: These mixtures have a uniform composition throughout, meaning the components are evenly distributed. Examples include saltwater and air.
- Heterogeneous mixtures: These mixtures have a non-uniform composition, meaning the components are not evenly distributed. Examples include sand and water, or a salad.
Baking Soda: Unpacking its Chemical Identity
Baking soda, also known as sodium bicarbonate, has the chemical formula NaHCO₃. This formula immediately provides a critical clue to its classification. The presence of chemically bonded sodium (Na), hydrogen (H), carbon (C), and oxygen (O) atoms indicates a specific and consistent arrangement.
The Chemical Bonds in Baking Soda
The atoms in sodium bicarbonate are held together by strong ionic and covalent bonds. Ionic bonds occur between the sodium ion (Na⁺) and the bicarbonate ion (HCO₃⁻), resulting from the electrostatic attraction between oppositely charged ions. Within the bicarbonate ion, covalent bonds connect the hydrogen, carbon, and oxygen atoms. These strong chemical bonds are the defining characteristic of a compound. It's not simply a mixture of these elements; they're fundamentally joined to form a new substance.
The Properties of Baking Soda: A Compound's Signature
Baking soda possesses unique properties distinctly different from its constituent elements. Sodium is a highly reactive metal, while hydrogen, carbon, and oxygen are gases. However, baking soda is a white crystalline powder that is relatively stable under normal conditions. This stark difference in properties is a strong indicator of a compound. The leavening action in baking, caused by the release of carbon dioxide when heated, is another example of a chemical property unique to the compound itself.
Separating the Components of Baking Soda: A Chemical Challenge
Attempting to separate the elements of baking soda using simple physical methods would be futile. You cannot simply filter out sodium, hydrogen, carbon, and oxygen from baking soda. To obtain these individual elements, you would require complex chemical processes, further demonstrating its compound nature.
Debunking the Mixture Misconception
Some might mistakenly consider baking soda a mixture due to its potential interaction with other ingredients, such as acids, in baking. However, these interactions are chemical reactions, not evidence of a mixture's inherent nature. Baking soda's reaction with an acid, such as vinegar or lemon juice, produces carbon dioxide gas and other products. This reaction demonstrates the compound's reactivity, not that it's a mixture. The chemical transformation is key—it’s not just a physical separation or combination.
Baking Soda: A Pure Compound, Not a Mixture
In conclusion, the evidence overwhelmingly supports the classification of baking soda (sodium bicarbonate) as a compound, not a mixture. Its fixed chemical formula (NaHCO₃), the presence of strong chemical bonds between its constituent elements, its unique physical and chemical properties distinct from those elements, and the necessity of chemical reactions for separation all firmly establish its status as a pure chemical compound.
Beyond the Basics: Exploring Related Concepts
Understanding the compound nature of baking soda opens doors to a broader understanding of chemical concepts. Let's briefly touch upon some related topics:
Molecular Structure and Bonding
The specific arrangement of atoms within the baking soda molecule (NaHCO₃) significantly influences its properties. The ionic and covalent bonds determine its stability, reactivity, and interactions with other substances. Exploring the molecular structure further reveals the intricate details of its chemical behavior.
Chemical Reactions and Baking
Baking soda's utility as a leavening agent hinges on its participation in acid-base reactions. When combined with an acid, it undergoes a chemical reaction, releasing carbon dioxide gas that helps baked goods rise. This understanding highlights the importance of chemical reactions in culinary applications.
Industrial Applications and Production
The industrial production of baking soda involves precise chemical processes that synthesize this compound. This underscores its importance not only in kitchens but also in various industrial settings.
The Importance of Chemical Nomenclature
The use of the systematic chemical name, sodium bicarbonate (NaHCO₃), rather than the common name "baking soda," is crucial in scientific contexts. This precise nomenclature ensures clarity and avoids confusion, particularly when dealing with various chemical compounds.
Conclusion: A Definitive Answer and Further Exploration
The question, "Is baking soda a compound or a mixture?", has a clear and definitive answer: baking soda is a compound. This understanding forms a solid foundation for a deeper exploration into chemistry, highlighting the fundamental differences between compounds and mixtures and their significance in various scientific and practical applications. From understanding chemical bonding to exploring the science of baking, baking soda serves as a fascinating example of the power and precision of chemical principles. Its seemingly simple presence in our kitchens opens a world of scientific discovery, proving that even everyday substances can hold remarkable complexity and intrigue.
Latest Posts
Latest Posts
-
Is Oil And Water A Homogeneous Mixture
Mar 17, 2025
-
How Are The Elements In The Modern Periodic Table Arranged
Mar 17, 2025
-
How Many Feet Is 7 5 M
Mar 17, 2025
-
1 4 Inch Is How Many Mm
Mar 17, 2025
-
What Is A Diaphragm In Microscope
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Baking Soda A Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
