Is Evaporation An Exothermic Or Endothermic Process
Kalali
Mar 10, 2025 · 5 min read
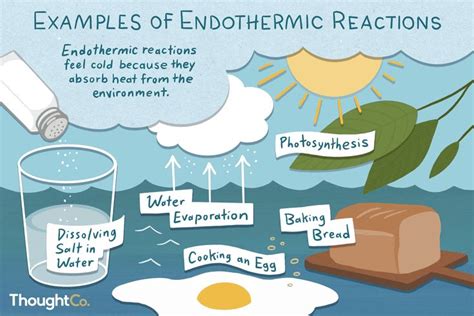
Table of Contents
Is Evaporation an Exothermic or Endothermic Process? A Deep Dive
Evaporation, a ubiquitous process shaping our climate and essential to life itself, often sparks the question: is it exothermic or endothermic? The simple answer is endothermic. But understanding why requires a deeper dive into the fundamental principles of thermodynamics and the molecular behavior of liquids. This comprehensive exploration will delve into the intricacies of evaporation, explaining its endothermic nature, exploring related concepts, and clarifying common misconceptions.
Understanding Endothermic and Exothermic Processes
Before we dissect evaporation, let's establish a clear understanding of endothermic and exothermic processes. These terms describe the energy exchange between a system and its surroundings during a reaction or phase transition.
-
Exothermic processes release energy into the surroundings, usually in the form of heat. The system's temperature increases, or the surroundings' temperature increases if the system is open. Examples include combustion (burning) and the neutralization of an acid with a base.
-
Endothermic processes absorb energy from the surroundings. The system's temperature decreases, or the surroundings' temperature decreases if the system is open. Examples include melting ice and photosynthesis.
The Molecular Dance of Evaporation: An Endothermic Affair
Evaporation is the phase transition where a liquid transforms into a gas. This transformation requires energy input, making it an endothermic process. Here's why:
1. Breaking Intermolecular Forces: The Energy Barrier
Liquid molecules are held together by intermolecular forces, such as van der Waals forces, hydrogen bonds, and dipole-dipole interactions. These forces vary in strength depending on the liquid's composition, influencing its boiling point and evaporation rate. For a molecule to escape the liquid phase and enter the gaseous phase, it must overcome these attractive forces. This requires energy. The energy is absorbed from the surroundings, cooling the remaining liquid.
2. Kinetic Energy and Escape Velocity: A Race Against Attraction
Molecules within a liquid possess kinetic energy, constantly moving and colliding. However, the intermolecular forces restrain their movement. Only molecules with sufficient kinetic energy can overcome these forces and escape into the gas phase. This is analogous to a rocket needing enough velocity to escape Earth's gravitational pull. The faster-moving, higher-energy molecules are the ones that evaporate, leaving behind the slower, lower-energy molecules. This selective escape of high-energy molecules results in a net decrease in the average kinetic energy of the remaining liquid, thus lowering its temperature.
3. Heat of Vaporization: Quantifying the Energy Demand
The amount of energy required to evaporate a specific quantity of a liquid at a constant temperature is called the heat of vaporization (or enthalpy of vaporization). This is a crucial parameter, varying for different substances based on their intermolecular forces. A higher heat of vaporization indicates stronger intermolecular forces and thus a greater energy requirement for evaporation. The heat of vaporization is always positive for endothermic processes, signifying energy absorption.
Factors Affecting Evaporation Rate: A Multifaceted Process
Several factors influence the rate at which evaporation occurs. These factors are all related to increasing the number of molecules with sufficient kinetic energy to overcome intermolecular forces.
1. Temperature: Heating Things Up
Higher temperatures lead to faster evaporation. Increased temperature translates to higher average kinetic energy among the liquid's molecules. Consequently, a larger fraction of molecules possess the necessary energy to escape the liquid phase.
2. Surface Area: Expanding the Escape Route
A larger surface area exposes more molecules to the surrounding environment, increasing the number of molecules that can evaporate simultaneously. Think of a puddle drying faster than a large lake – the higher surface area-to-volume ratio of the puddle accelerates evaporation.
3. Airflow: Clearing the Path
Moving air removes evaporated molecules from the vicinity of the liquid's surface. This prevents the re-condensation of vapor molecules back into the liquid, facilitating continuous evaporation. A breeze accelerates the drying of clothes, for instance.
4. Humidity: The Vapor Pressure Influence
High humidity (high concentration of water vapor in the air) reduces the evaporation rate. The air already contains a significant amount of water vapor, limiting the space available for additional molecules to enter the gaseous phase. Conversely, low humidity allows for faster evaporation.
Common Misconceptions about Evaporation
Several misconceptions surround evaporation, which we will address here:
-
Evaporation is cooling because the liquid is losing heat: While the liquid's temperature decreases during evaporation, it's more accurate to say it's because the fastest molecules are leaving, taking their kinetic energy with them. The heat isn’t necessarily lost, but rather redistributed.
-
Evaporation only happens at high temperatures: While higher temperatures accelerate evaporation, it can occur at any temperature above the liquid's freezing point. Even on a cold day, a small amount of evaporation is always taking place.
-
Evaporation is always a rapid process: The rate of evaporation varies significantly based on the factors discussed above. Some liquids evaporate quickly, while others evaporate slowly.
Evaporation in Everyday Life and Beyond: A Ubiquitous Phenomenon
Evaporation is not just a classroom concept; it plays a crucial role in numerous natural and technological processes:
-
Weather patterns: Evaporation from water bodies drives the water cycle, contributing to cloud formation and precipitation.
-
Cooling mechanisms: Sweating cools the human body through evaporative heat loss. Similarly, evaporative coolers utilize water evaporation to lower air temperature.
-
Industrial processes: Evaporation is employed in various industrial applications, such as drying materials and concentrating solutions.
-
Biological systems: Plants utilize transpiration (evaporation from leaves) to transport water and nutrients.
Conclusion: A Fundamental Endothermic Process
In conclusion, evaporation is unequivocally an endothermic process. It requires energy input to overcome intermolecular forces and enable molecules to transition from the liquid to the gaseous phase. This energy is absorbed from the surroundings, leading to a decrease in the liquid's temperature. Understanding the endothermic nature of evaporation provides a foundation for comprehending various natural phenomena and technological applications. The factors affecting evaporation rate, along with clarifying common misconceptions, offer a holistic perspective on this vital process that shapes our world. From the gentle cooling of a sweating brow to the vast scale of global weather patterns, evaporation remains a fundamental and fascinating aspect of our physical reality.
Latest Posts
Latest Posts
-
The Shaft Of A Long Bone Is Known As The
Mar 11, 2025
-
Where Is The Sugar Removed From The Blood
Mar 11, 2025
-
1 3 4 As A Decimal
Mar 11, 2025
-
Gas Below Krypton On The Periodic Table
Mar 11, 2025
-
159 Cm In Feet And Inches
Mar 11, 2025
Related Post
Thank you for visiting our website which covers about Is Evaporation An Exothermic Or Endothermic Process . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
