Is Kcl An Acid Or Base
Kalali
Mar 12, 2025 · 5 min read
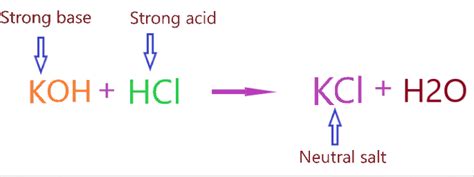
Table of Contents
Is KCl an Acid or a Base? Understanding Salt Hydrolysis
Potassium chloride (KCl), a common salt found in various applications, often sparks the question: is it an acid or a base? The answer isn't a simple "yes" or "no," but rather a nuanced understanding of its behavior in solution and the concept of salt hydrolysis. This comprehensive guide will delve deep into the properties of KCl, explore the principles of acid-base chemistry, and ultimately clarify its classification.
Understanding Acids, Bases, and Salts
Before classifying KCl, let's solidify our understanding of acids, bases, and how salts are formed.
Acids: Proton Donors
Acids are substances that donate protons (H⁺ ions) when dissolved in water. Strong acids, like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), completely dissociate, releasing all their protons. Weak acids, such as acetic acid (CH₃COOH), only partially dissociate, maintaining an equilibrium between the undissociated acid and its ions. The strength of an acid is determined by its tendency to donate protons.
Bases: Proton Acceptors
Bases are substances that accept protons (H⁺ ions) when dissolved in water. Strong bases, like sodium hydroxide (NaOH) and potassium hydroxide (KOH), completely dissociate into their ions. Weak bases, such as ammonia (NH₃), only partially accept protons, establishing an equilibrium. The strength of a base is determined by its affinity for protons.
Salts: Products of Acid-Base Reactions
Salts are ionic compounds formed from the reaction between an acid and a base. This reaction, called neutralization, involves the combination of H⁺ ions from the acid and OH⁻ ions from the base to form water (H₂O). The remaining ions from the acid and base combine to form the salt. For example, the reaction between HCl (acid) and NaOH (base) produces NaCl (salt) and H₂O (water).
KCl: A Neutral Salt?
KCl is formed from the reaction between a strong acid (HCl) and a strong base (KOH):
HCl + KOH → KCl + H₂O
Since KCl is formed from a strong acid and a strong base, it is considered a neutral salt. This means that in pure water, it doesn't significantly affect the pH. However, this neutrality isn't absolute and requires a more in-depth explanation.
Salt Hydrolysis: The Key to Understanding KCl's Behavior
The apparent neutrality of KCl stems from the fact that neither the K⁺ ion nor the Cl⁻ ion undergoes significant hydrolysis. Hydrolysis is the reaction of a salt with water to produce an acidic or basic solution.
Let's examine each ion individually:
Potassium Ion (K⁺): A Spectator Ion
The potassium ion (K⁺) is the conjugate acid of the strong base KOH. Conjugate acids of strong bases are very weak acids. This means that K⁺ has virtually no tendency to react with water to produce H⁺ ions. It essentially remains a spectator ion, meaning it doesn't significantly participate in the acid-base equilibrium of the solution.
Chloride Ion (Cl⁻): A Spectator Ion
Similarly, the chloride ion (Cl⁻) is the conjugate base of the strong acid HCl. Conjugate bases of strong acids are very weak bases. Therefore, Cl⁻ has negligible tendency to react with water to produce OH⁻ ions. It too acts as a spectator ion.
Why KCl Doesn't Significantly Affect pH
Because both K⁺ and Cl⁻ are extremely weak in their conjugate acid-base properties, they do not significantly alter the concentration of H⁺ or OH⁻ ions in water. Consequently, the pH of a KCl solution remains very close to 7 (neutral).
In essence, the neutrality of a KCl solution arises from the weak conjugate acid and base nature of its constituent ions, preventing substantial hydrolysis.
Factors Affecting KCl's Apparent Neutrality
While KCl is generally considered a neutral salt, minor deviations from a perfectly neutral pH can occur under specific conditions. These factors include:
-
Impurities: The presence of impurities in the KCl sample can affect its pH. Contaminants with acidic or basic properties can alter the overall pH of the solution. High-purity KCl is crucial for maintaining neutrality.
-
Temperature: The dissociation of water (and hence the pH) is slightly temperature-dependent. Slight variations in temperature can influence the apparent pH of a KCl solution.
-
Concentration: While high concentrations generally don't drastically alter the pH of KCl solutions, extremely high concentrations might introduce slight deviations due to ionic strength effects.
Applications of KCl: Leveraging its Neutral Nature
KCl's neutral nature makes it suitable for a wide range of applications where maintaining a neutral pH is crucial:
-
Electrolyte Solutions: KCl solutions are frequently used in biological experiments and medical applications as electrolytes because of their negligible influence on pH. This property is essential to prevent interference with biological processes.
-
Food Additives: KCl serves as a salt substitute in food, providing a salty flavor without the negative health consequences associated with excessive sodium intake. Its neutrality ensures it doesn't negatively affect food taste or chemistry.
-
Fertilizers: KCl is a valuable source of potassium, an essential nutrient for plant growth. Its neutral nature ensures soil pH isn't significantly altered, preventing harm to plants.
-
Medical Applications: KCl plays vital roles in intravenous solutions and medical treatments. Its neutral pH helps avoid adverse reactions when introduced into the body.
Conclusion: KCl – A Neutral Salt with Practical Significance
In conclusion, KCl is appropriately classified as a neutral salt. The weak conjugate acid and base properties of its constituent ions, K⁺ and Cl⁻, prevent significant hydrolysis in aqueous solutions, resulting in a pH very close to 7. While minor variations in pH might occur due to factors like impurities or temperature, KCl's overall neutrality is a key characteristic that dictates its widespread applications in various scientific, industrial, and medical fields. Understanding the principles of acid-base chemistry and salt hydrolysis is crucial for appreciating the behavior and practical utility of this common yet important compound. The seemingly simple question of whether KCl is an acid or a base highlights the complexities and subtleties inherent in understanding chemical behavior.
Latest Posts
Latest Posts
-
A Horizontal Row Of Elements In The Periodic Table
Mar 12, 2025
-
Does A Wedge Increases The Distance
Mar 12, 2025
-
What Number Is 15 Of 30
Mar 12, 2025
-
How Does Biosphere Interact With Atmosphere
Mar 12, 2025
-
Mechanical Advantage Formula On A Seesaw
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Is Kcl An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
