Is Pure Water Homogeneous Or Heterogeneous
Kalali
Mar 29, 2025 · 5 min read
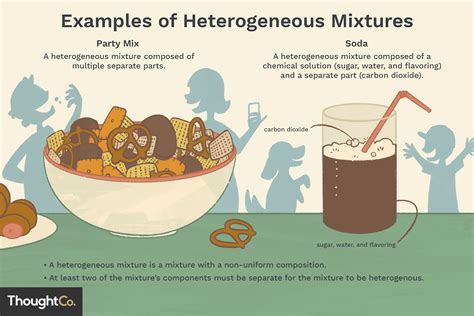
Table of Contents
Is Pure Water Homogeneous or Heterogeneous? A Deep Dive into Matter Classification
The question of whether pure water is homogeneous or heterogeneous might seem straightforward, but it delves into fundamental concepts of chemistry and the nature of matter. Understanding the difference between homogeneous and heterogeneous mixtures is crucial for various scientific disciplines, from material science to environmental studies. This comprehensive article will explore the characteristics of pure water, dissect the definitions of homogeneous and heterogeneous mixtures, and definitively answer the question while exploring related concepts.
Understanding Homogeneous and Heterogeneous Mixtures
Before we dive into the specifics of water, let's establish a clear understanding of the terminology:
Homogeneous Mixture: A homogeneous mixture is a type of mixture in which the composition is uniform throughout the mixture. This means that at the macroscopic level (what you can see with the naked eye or even a standard microscope), the different components are indistinguishable. The properties of the mixture are consistent regardless of the sample location. Think of saltwater: once the salt is fully dissolved, you can't visually distinguish the salt from the water. The concentration of salt is consistent throughout the solution. Other examples include air (a mixture of gases) and many metal alloys.
Heterogeneous Mixture: In contrast, a heterogeneous mixture has a non-uniform composition. Different components are visibly distinguishable, and the properties of the mixture vary depending on the location of the sample. Think of a salad: you can easily see and distinguish the lettuce, tomatoes, and cucumbers. The composition is not uniform throughout. Other examples include sand and water, oil and water, and a chocolate chip cookie.
The Nature of Pure Water
Pure water, in its simplest form, is composed solely of water molecules (H₂O). These molecules are formed by covalent bonds between two hydrogen atoms and one oxygen atom. Critically, pure water does not contain any other substances dissolved or suspended within it. This absence of impurities is key to understanding its classification.
Key Characteristics of Pure Water
- Molecular Composition: Consists entirely of H₂O molecules.
- Lack of Impurities: Free from dissolved solids, gases, or other substances.
- Transparency: Clear and colorless (though it can appear slightly blue in large volumes due to light absorption).
- Uniform Properties: Its properties, such as density, pH (approximately 7), and refractive index, are consistent throughout the sample.
Is Pure Water Homogeneous or Heterogeneous? The Definitive Answer
Given the characteristics of pure water and the definitions of homogeneous and heterogeneous mixtures, the answer is clear: Pure water is a homogeneous mixture.
The reason is its uniformity. At the molecular level, while individual water molecules interact, there is no macroscopic segregation or separation of distinct components. Every sample of pure water, regardless of its volume or location of sampling, will exhibit identical physical and chemical properties. This consistency of composition across the entire sample is the defining characteristic of a homogeneous mixture.
Common Misconceptions and Clarifications
It's important to address some common misunderstandings:
1. The Presence of Isotopes:
Water molecules can contain different isotopes of hydrogen (protium, deuterium, tritium) and oxygen (¹⁶O, ¹⁷O, ¹⁸O). However, the distribution of these isotopes is generally random and does not create a macroscopic non-uniformity. While the isotopic composition might slightly vary geographically, the difference is negligible at the scale of typical observation and does not classify pure water as heterogeneous.
2. Water from Different Sources:
Water collected from different sources (e.g., a river, a well, rainwater) will inevitably contain dissolved impurities. This is not pure water. Pure water, as we're defining it here, is distilled or otherwise purified to remove all impurities. Impure water from natural sources is, in fact, often heterogeneous due to the presence of suspended sediments, dissolved minerals, and microorganisms.
3. Microscopic Variations:
At an extremely microscopic scale, there might be minuscule local variations in density or molecular arrangement within pure water. However, these fluctuations are statistically insignificant and do not contradict the macroscopic homogeneity of the substance. The properties remain uniform at scales relevant to typical observation and measurement.
The Significance of Homogeneity in Scientific Applications
The homogeneity of pure water is crucial in many scientific and technological applications:
- Calibration and Standardization: Pure water serves as a standard in various analytical techniques, such as spectrophotometry and chromatography, requiring a consistent and uniform composition for accurate measurements.
- Laboratory Experiments: Many experiments require pure water to avoid the interference of impurities, ensuring reproducible results and precise control of experimental conditions.
- Pharmaceutical and Medical Applications: Pure water is essential in the preparation of pharmaceuticals and intravenous solutions, where any contamination can have serious health consequences.
- Industrial Processes: Various industrial processes use pure water as a solvent, reagent, or coolant, relying on its consistent properties for optimal performance and efficiency.
Distinguishing Pure Water from Impure Water: Practical Considerations
It's vital to emphasize that the term "pure water" is an idealization. In practice, achieving perfectly pure water is exceptionally challenging. Even highly purified water will contain trace impurities at the parts-per-billion level. However, for most practical purposes, water with extremely low levels of impurities is considered "pure" enough for the intended application.
Several techniques are used to purify water, including:
- Distillation: Separates water from impurities based on their boiling points.
- Reverse Osmosis: Uses pressure to force water through a semi-permeable membrane, removing dissolved solids.
- Deionization: Removes ions from water using ion exchange resins.
The degree of purity required depends on the specific application. For laboratory experiments demanding high precision, highly purified water is necessary. However, for everyday uses, the level of purity can be less stringent.
Conclusion: Understanding the Nuances of Mixture Classification
This in-depth analysis demonstrates that pure water, devoid of any dissolved or suspended substances, is a homogeneous mixture. Its uniform composition at the macroscopic level aligns perfectly with the definition of homogeneity. Understanding this distinction is essential for various scientific applications and highlights the importance of precise terminology in scientific discourse. The concept of purity, while idealized, is crucial for many practical applications, emphasizing the importance of water purification techniques in achieving the desired level of homogeneity for specific tasks. The nuances between pure and impure water underscore the complexity of classifying matter and the need for a clear understanding of these fundamental concepts.
Latest Posts
Latest Posts
-
3 Of 16 Is What Percent
Mar 31, 2025
-
What Is 375 Degrees Fahrenheit In Centigrade
Mar 31, 2025
-
Cuanto Es El 1 Por Ciento De 1000
Mar 31, 2025
-
Gravitational Force Exerted On An Object
Mar 31, 2025
-
A Diatomic Molecule With A Triple Covalent Bond Is
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Pure Water Homogeneous Or Heterogeneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
