What Is The Molecular Geometry Of Ozone O3
Kalali
Mar 27, 2025 · 5 min read
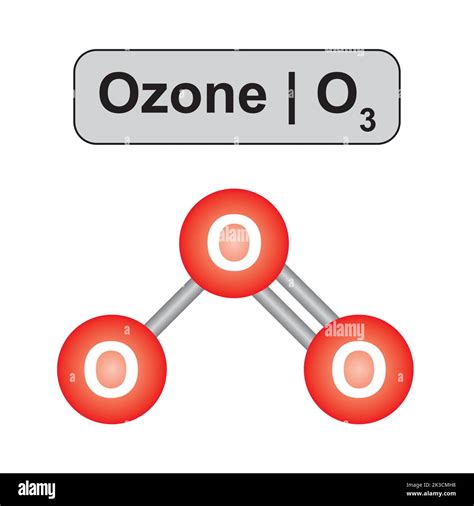
Table of Contents
What is the Molecular Geometry of Ozone (O3)?
Ozone (O3), a crucial component of the Earth's atmosphere, possesses a fascinating molecular geometry that significantly influences its chemical properties and reactivity. Understanding its structure is key to comprehending its role in atmospheric chemistry, its impact on human health, and its various applications. This in-depth article delves into the molecular geometry of ozone, exploring its bonding, bond angles, resonance structures, and the factors contributing to its unique shape.
Understanding Molecular Geometry: VSEPR Theory
Before diving into the specifics of ozone's geometry, it's essential to understand the fundamental principles governing molecular shapes. The Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone in predicting molecular geometries. This theory posits that electron pairs, both bonding and non-bonding (lone pairs), arrange themselves around a central atom to minimize electrostatic repulsion. This arrangement dictates the molecule's overall shape.
Key Concepts in VSEPR Theory:
- Electron Domains: These represent regions of high electron density around a central atom. They can be either bonding pairs (involved in covalent bonds) or lone pairs (unshared electrons).
- Minimizing Repulsion: Electron domains orient themselves as far apart as possible to minimize the repulsive forces between them.
- Steric Number: This refers to the total number of electron domains surrounding the central atom (bonding pairs + lone pairs).
Ozone's Lewis Structure and Electron Domains
Ozone's Lewis structure reveals the arrangement of valence electrons in the molecule. Oxygen has six valence electrons. In O3, a total of 18 valence electrons are involved (3 oxygen atoms x 6 valence electrons/atom).
A possible Lewis structure shows a central oxygen atom double-bonded to one oxygen atom and single-bonded to another. This arrangement leaves one lone pair on the central oxygen and three lone pairs on the terminal oxygen atoms.
However, this structure is not the complete picture. Due to the resonance effect described below, the actual structure is a hybrid.
Resonance Structures of Ozone
The true representation of ozone's bonding involves resonance structures. This means that the actual electron distribution is a hybrid of multiple contributing Lewis structures. For ozone, two equivalent resonance structures can be drawn:
O=O-O <-> O-O=O
In each structure, one oxygen atom carries a formal negative charge, while another carries a formal positive charge. The actual structure is a resonance hybrid, where the double bond is delocalized across both O-O bonds. This means the bond length between the oxygen atoms is intermediate between a single and a double bond.
Determining Ozone's Molecular Geometry
Applying the VSEPR theory to ozone (considering the resonance hybrid), we find the following:
- Central Atom: The central oxygen atom.
- Electron Domains: The central oxygen atom has three electron domains: two bonding pairs and one lone pair.
- Steric Number: The steric number is 3.
- Predicted Geometry: Based on VSEPR theory, three electron domains around a central atom result in a trigonal planar electron domain geometry. However, the presence of a lone pair affects the molecular geometry.
Ozone's Molecular Geometry: Bent Shape
Because of the lone pair on the central oxygen atom, the molecular geometry of ozone is bent or angular, rather than perfectly trigonal planar. The lone pair occupies more space than a bonding pair, causing a slight compression of the O-O-O bond angle.
Bond Angle in Ozone
The O-O-O bond angle in ozone is approximately 117 degrees. This is slightly less than the ideal 120-degree angle expected for a trigonal planar arrangement. The deviation is attributed to the lone pair's greater repulsive force compared to the bonding pairs.
Polarity of the Ozone Molecule
The bent shape of ozone, combined with the difference in electronegativity between oxygen atoms (although they are the same element, the charge distribution due to resonance makes this important to consider), results in a polar molecule. The unequal distribution of electron density leads to a net dipole moment. This polarity plays a significant role in ozone's interactions with other molecules and its reactivity.
Significance of Ozone's Molecular Geometry
The bent geometry and polar nature of ozone have profound implications:
- Atmospheric Chemistry: Ozone's ability to absorb ultraviolet (UV) radiation is directly related to its molecular structure. The bent geometry and polar nature influence its interactions with other atmospheric molecules, playing a critical role in ozone depletion and formation.
- Reactivity: The polar nature of ozone enhances its reactivity, making it a strong oxidizing agent. This property is used in various applications, such as water purification and disinfection.
- Spectroscopic Properties: Ozone's molecular geometry and electronic structure influence its infrared and UV-Vis spectral characteristics, making it identifiable through spectroscopic techniques.
Advanced Considerations: Molecular Orbital Theory
While VSEPR theory provides a simple and effective model for predicting molecular geometry, a more sophisticated approach, molecular orbital (MO) theory, offers a deeper understanding of ozone's bonding. MO theory describes the formation of molecular orbitals from the combination of atomic orbitals. In ozone, the combination of oxygen atomic orbitals results in bonding and antibonding molecular orbitals, ultimately leading to a delocalized pi system above and below the plane of the molecule. This further clarifies the resonance effect observed in the Lewis structures.
Conclusion: The Importance of Understanding Ozone's Geometry
The molecular geometry of ozone, a bent structure resulting from three electron domains around the central oxygen atom and the influence of resonance, significantly impacts its physical and chemical properties. Understanding this geometry is fundamental to comprehending its critical role in atmospheric processes, its reactivity, and its various applications. From its absorption of harmful UV radiation to its oxidizing power, ozone's unique structure underpins its significant influence on our environment and technological advancements. Further investigation into its molecular geometry continues to be a subject of scientific study, leading to better understanding of its behavior and implications for the environment and human health. The combination of VSEPR and MO theory offers a complete description of the complex but essential structure of this important molecule. The interplay between simple models like VSEPR and more advanced theoretical approaches like MO theory highlights the power of diverse perspectives in comprehending the complexities of molecular structure and function.
Latest Posts
Latest Posts
-
What Is A Positive Ion Called
Mar 30, 2025
-
How Many Liters In 64 Oz
Mar 30, 2025
-
What Does Mu Mean In Statistics
Mar 30, 2025
-
What Type Of Organism Is The Grass
Mar 30, 2025
-
How Much Is 2 3 Cup In Ounces
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Geometry Of Ozone O3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
