What Is The Solvent In Salt Water
Kalali
Mar 27, 2025 · 6 min read
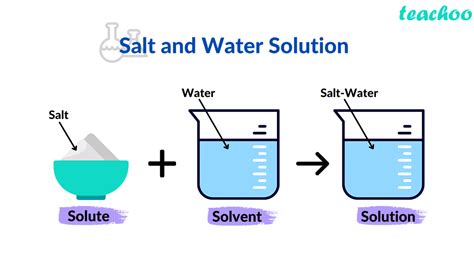
Table of Contents
What is the Solvent in Salt Water? A Deep Dive into Solutions
Understanding the components of a solution is fundamental to chemistry. When we talk about saltwater, a seemingly simple mixture, the question of which component acts as the solvent becomes surprisingly nuanced. This article delves into the definition of a solvent, explores the properties of saltwater, and definitively answers the question: what is the solvent in saltwater?
Defining Solvents and Solutes
Before we dissect saltwater, let's clarify the terms. A solution is a homogeneous mixture of two or more substances. These substances are classified as either a solvent or a solute.
-
Solvent: This is the substance that dissolves the other substance(s). It is typically present in the larger quantity. Think of it as the "doing the dissolving." The solvent determines the physical state of the solution. For example, if the solvent is liquid, the solution will also be liquid.
-
Solute: This is the substance that is dissolved by the solvent. It is typically present in the smaller quantity. The solute is the substance that undergoes a change in state.
The Composition of Saltwater
Saltwater, as the name suggests, is a solution composed primarily of water and salt (sodium chloride, NaCl). Let's examine each component:
-
Water (H₂O): Water is a polar molecule, meaning it has a slightly positive end and a slightly negative end. This polarity allows water to interact effectively with ionic compounds like salt.
-
Salt (NaCl): Salt is an ionic compound, meaning it's composed of positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). The strong electrostatic forces holding these ions together in the crystal lattice are overcome by the polar water molecules.
The Dissolving Process in Saltwater
The dissolving of salt in water is a fascinating process involving several steps:
-
Hydration: When salt is added to water, the polar water molecules surround the Na⁺ and Cl⁻ ions. The slightly negative oxygen atoms in water molecules are attracted to the positive sodium ions, while the slightly positive hydrogen atoms are attracted to the negative chloride ions. This process is called hydration.
-
Ion-Dipole Interactions: The attraction between the charged ions and the polar water molecules is known as ion-dipole interactions. These interactions are strong enough to overcome the electrostatic forces holding the salt crystal together.
-
Dissociation: As the water molecules surround the ions, the ionic bonds in the salt crystal are broken, and the individual ions become separated and surrounded by water molecules. This process is called dissociation.
-
Homogeneous Mixture: The hydrated ions are evenly distributed throughout the water, resulting in a homogeneous mixture—saltwater.
Identifying the Solvent in Saltwater
Given the definitions and the dissolving process, the answer is clear: water is the solvent in saltwater.
Here's why:
-
Quantity: Water is present in a significantly larger quantity than salt in saltwater. Ocean water, for example, contains around 3.5% salt by weight. The vast majority of the solution is water.
-
Dissolving Agent: Water actively dissolves the salt. The polar water molecules surround and separate the sodium and chloride ions, enabling them to disperse uniformly throughout the solution. The salt, on the other hand, does not dissolve water.
-
State of the Solution: The physical state of saltwater is liquid, mirroring the liquid state of the water.
Misconceptions about Solvents
It's crucial to dispel some common misconceptions about solvents:
-
The solvent isn't always the component in larger quantity: While usually true, this isn't a strict rule. In some specialized situations, such as very concentrated solutions, the quantity alone may not definitively identify the solvent. However, in everyday scenarios, like saltwater, quantity is a reliable indicator.
-
The solvent doesn't need to be a liquid: Solvents can exist in other states. For instance, in alloys (solid solutions), one metal acts as a solvent for another. Similarly, gases can dissolve in other gases. However, in the case of saltwater, both the solute (salt) and solvent (water) are initially in different states, making the solvent definitively liquid.
Applications of Understanding Solvents and Solutions
The ability to identify the solvent in a solution is crucial in various applications:
-
Environmental Science: Understanding how solvents interact with pollutants and other substances is vital for water treatment, pollution control, and environmental remediation.
-
Medicine: The properties of solvents are critical in the development and administration of drugs and medications. Many drugs are dissolved in solvents to facilitate absorption and delivery to the body.
-
Chemistry and Material Science: Choosing the appropriate solvent is critical in many chemical reactions and processes. The solvent can influence reaction rates, product yields, and the properties of the resulting materials.
-
Food Science: Many food processing techniques involve dissolving substances in solvents. For example, extracting flavors or colors from natural sources often involves using solvents.
Exploring Different Types of Solutions
Beyond saltwater, understanding the concepts of solvents and solutes is important when considering different types of solutions:
-
Aqueous Solutions: These are solutions where water is the solvent. Saltwater is a classic example, but many other substances, including sugars, acids, and bases, dissolve readily in water.
-
Non-Aqueous Solutions: These solutions use solvents other than water. Common examples include solutions using ethanol, benzene, or other organic solvents.
-
Saturated Solutions: This occurs when the maximum amount of solute has dissolved in a given solvent at a specific temperature and pressure. Adding more solute won't result in any further dissolving.
-
Unsaturated Solutions: These solutions have less than the maximum amount of solute dissolved in the solvent.
-
Supersaturated Solutions: These solutions contain more solute than can typically be dissolved at a given temperature and pressure. These are usually unstable and easily revert to saturated solutions.
Advanced Concepts: Solubility and Polarity
The ability of a solvent to dissolve a solute depends heavily on the solubility of the solute in the solvent. Solubility is affected by several factors, including temperature, pressure, and the polarity of both the solute and the solvent.
-
Polar Solvents: Like water, polar solvents dissolve polar solutes and ionic compounds effectively. The strong electrostatic interactions between the polar solvent molecules and the solute particles lead to dissolution.
-
Nonpolar Solvents: Nonpolar solvents, such as oil or benzene, dissolve nonpolar solutes. The "like dissolves like" rule highlights the importance of matching the polarities of the solvent and solute for effective dissolution.
Conclusion: The Definitive Answer
In conclusion, the solvent in saltwater is definitively water. The polar nature of water molecules allows them to effectively interact with the ionic sodium and chloride ions in salt, leading to the dissolution and formation of a homogeneous solution. Understanding this fundamental concept is essential for comprehending a wide range of chemical and physical processes. The principles of solvents and solutes extend far beyond saltwater, impacting numerous scientific fields and everyday applications. By grasping these concepts, we can better understand the world around us and apply this knowledge to various practical situations.
Latest Posts
Latest Posts
-
What Is A Positive Ion Called
Mar 30, 2025
-
How Many Liters In 64 Oz
Mar 30, 2025
-
What Does Mu Mean In Statistics
Mar 30, 2025
-
What Type Of Organism Is The Grass
Mar 30, 2025
-
How Much Is 2 3 Cup In Ounces
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Solvent In Salt Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
