Which Of The Following Is Considered A Strong Electrolyte
Kalali
Mar 19, 2025 · 6 min read
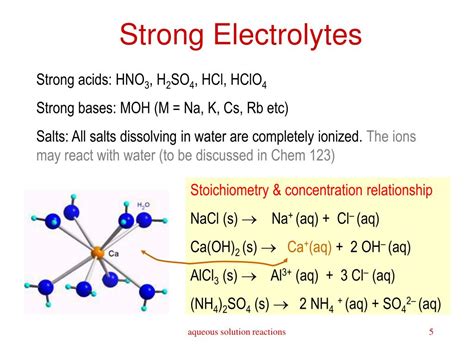
Table of Contents
Which of the Following is Considered a Strong Electrolyte? Understanding Electrolyte Strength and Behavior
The question of which substance qualifies as a strong electrolyte hinges on understanding the fundamental concept of electrolyte strength and its implications in solution chemistry. Electrolytes are substances that, when dissolved in a suitable solvent (typically water), produce a solution that conducts electricity. This conductivity arises from the presence of mobile ions—charged particles—capable of carrying an electric current. The strength of an electrolyte is determined by the extent to which it dissociates into these ions. Let's delve into the details, exploring what makes a strong electrolyte, the differences between strong and weak electrolytes, and how to identify them.
Strong Electrolytes: Complete Dissociation and High Conductivity
A strong electrolyte is a substance that essentially completely dissociates into its constituent ions when dissolved in water. This means that virtually every molecule of the solute breaks apart into ions. Consequently, strong electrolyte solutions exhibit high electrical conductivity. The more ions present, the better the solution conducts electricity. This complete dissociation leads to a high concentration of ions, maximizing the current carrying capacity.
Key Characteristics of Strong Electrolytes:
- Complete dissociation: Nearly 100% of the solute dissociates into ions.
- High conductivity: Solutions are excellent conductors of electricity.
- High concentration of ions: The solution contains a large number of mobile charge carriers.
- Irreversible dissociation: The dissociation process is essentially unidirectional; the ions don't readily recombine to reform the neutral molecule.
Weak Electrolytes: Partial Dissociation and Low Conductivity
In contrast, a weak electrolyte only partially dissociates in water. A significant portion of the solute remains in its molecular form, with only a small fraction breaking down into ions. This limited dissociation results in low electrical conductivity. The fewer the ions present, the less effectively the solution conducts electricity. The equilibrium between the undissociated molecules and the ions plays a crucial role in determining the solution's conductivity.
Key Characteristics of Weak Electrolytes:
- Partial dissociation: Only a small percentage of the solute dissociates into ions.
- Low conductivity: Solutions are poor conductors of electricity.
- Low concentration of ions: The solution contains a relatively small number of mobile charge carriers.
- Equilibrium between ions and molecules: A dynamic equilibrium exists between the undissociated molecules and their constituent ions.
Identifying Strong and Weak Electrolytes: Rules and Examples
Identifying whether a substance is a strong or weak electrolyte often involves understanding its chemical nature. Here are some general rules:
Strong Electrolytes Typically Include:
- Most soluble salts: Salts formed from the reaction of a strong acid and a strong base generally dissociate completely in water. Examples include NaCl (sodium chloride), KCl (potassium chloride), and NaNO₃ (sodium nitrate).
- Strong acids: These acids completely ionize in water, releasing H⁺ ions. Examples include HCl (hydrochloric acid), H₂SO₄ (sulfuric acid), HNO₃ (nitric acid), HI (hydroiodic acid), HBr (hydrobromic acid), and HClO₄ (perchloric acid).
- Strong bases: These bases completely dissociate in water, releasing OH⁻ ions. Examples include NaOH (sodium hydroxide), KOH (potassium hydroxide), LiOH (lithium hydroxide), Ca(OH)₂ (calcium hydroxide), Sr(OH)₂ (strontium hydroxide), and Ba(OH)₂ (barium hydroxide).
Weak Electrolytes Typically Include:
- Weak acids: These acids only partially ionize in water, releasing a small amount of H⁺ ions. Examples include CH₃COOH (acetic acid), HF (hydrofluoric acid), H₂CO₃ (carbonic acid), and many organic acids.
- Weak bases: These bases only partially dissociate in water, releasing a small amount of OH⁻ ions. Examples include NH₃ (ammonia) and many organic amines.
- Many molecular compounds: Many covalent compounds do not dissociate into ions when dissolved in water and therefore are not electrolytes. Examples include sugar (sucrose) and ethanol.
Factors Affecting Electrolyte Strength
Several factors can influence the strength of an electrolyte:
- Nature of the solute: The chemical structure and bonding within the solute molecule determine its tendency to dissociate. Ionic compounds generally form strong electrolytes, while many covalent compounds form weak electrolytes or are nonelectrolytes.
- Solvent polarity: The polarity of the solvent plays a significant role in the dissociation process. Polar solvents like water effectively solvate ions, stabilizing them and promoting dissociation. Nonpolar solvents generally do not facilitate the dissociation of ionic compounds.
- Temperature: Increasing the temperature usually increases the degree of dissociation for weak electrolytes, leading to slightly higher conductivity.
Practical Applications of Electrolyte Strength
The distinction between strong and weak electrolytes has crucial implications in various fields:
- Electrochemistry: Strong electrolytes are essential components in batteries, fuel cells, and other electrochemical devices, providing the ions needed to carry the electric current.
- Medicine: Electrolytes such as sodium, potassium, calcium, and chloride ions are vital for maintaining proper physiological function. Imbalances in electrolyte levels can lead to serious health problems.
- Industrial processes: Electrolyte solutions are widely used in various industrial processes, such as electroplating, metal refining, and water treatment.
- Analytical chemistry: The conductivity of solutions is used in analytical chemistry to determine the concentration of electrolytes and to monitor reactions.
Examples and Comparisons: Determining Strong Electrolyte Status
Let's consider some specific examples to illustrate the concept:
Example 1: Compare NaCl (sodium chloride) and CH₃COOH (acetic acid).
NaCl is a strong electrolyte because it completely dissociates in water into Na⁺ and Cl⁻ ions. Acetic acid, on the other hand, is a weak electrolyte because only a small fraction of its molecules dissociate into CH₃COO⁻ and H⁺ ions. The conductivity of a NaCl solution will be significantly higher than that of an acetic acid solution of the same concentration.
Example 2: Consider HCl (hydrochloric acid) and HF (hydrofluoric acid).
HCl is a strong acid and a strong electrolyte, completely dissociating into H⁺ and Cl⁻ ions. HF, while an acid, is a weak acid and a weak electrolyte, only partially dissociating into H⁺ and F⁻ ions. The difference lies in the strength of the H-F bond, which is stronger than the H-Cl bond, making it less likely to ionize completely.
Example 3: Compare NaOH (sodium hydroxide) and NH₃ (ammonia).
NaOH is a strong base and a strong electrolyte, fully dissociating into Na⁺ and OH⁻ ions. NH₃, while a base, is a weak base and a weak electrolyte, only partially reacting with water to form a small amount of NH₄⁺ and OH⁻ ions. The difference arises from the relative ability of these substances to donate hydroxide ions (OH⁻) to the solution.
Conclusion: Understanding Electrolyte Strength for Effective Problem Solving
The ability to distinguish between strong and weak electrolytes is fundamental to understanding solution chemistry and its various applications. Remembering the key characteristics—complete versus partial dissociation and the resulting conductivity differences—allows for accurate prediction of solution behavior and effective problem-solving in diverse scientific and engineering contexts. By understanding the factors influencing electrolyte strength, we can better comprehend the behavior of solutions and apply this knowledge to various practical applications. The examples provided serve as a useful guide for identifying strong electrolytes and contrasting them with their weak counterparts.
Latest Posts
Latest Posts
-
Elements That Can Conduct Electricity Are Called
Mar 19, 2025
-
Cuanto Es El 15 Porciento De 1000
Mar 19, 2025
-
How Did The Internet Transform Scientific Research
Mar 19, 2025
-
What Is 1 3 Of 100 Percent
Mar 19, 2025
-
Supports Combustion Physical Or Chemical Property
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Considered A Strong Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
