Which Process Reduces Molecular Oxygen To Water
Kalali
Mar 17, 2025 · 5 min read
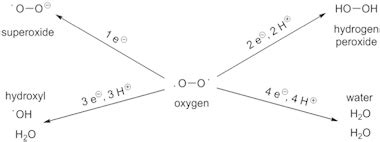
Table of Contents
Which Process Reduces Molecular Oxygen to Water?
The reduction of molecular oxygen (O₂) to water (H₂O) is a crucial process in various biological and chemical systems. Understanding this reduction is fundamental to comprehending respiration, photosynthesis, and various industrial processes. This article will delve deep into the mechanisms involved, exploring the different pathways and factors influencing the reduction of O₂ to H₂O.
The Importance of Oxygen Reduction
The reduction of oxygen to water is a fundamental redox reaction with immense significance across multiple disciplines:
Biological Significance: Respiration
Cellular respiration, the process by which organisms derive energy from organic molecules, hinges on the reduction of oxygen. In the electron transport chain (ETC) located within the mitochondria, electrons are passed along a series of protein complexes, ultimately reducing oxygen to water. This process generates a proton gradient, which is then used by ATP synthase to produce ATP, the primary energy currency of the cell. Without this oxygen reduction step, the ETC would halt, and cellular respiration would cease.
Biological Significance: Photosynthesis
While photosynthesis primarily involves the oxidation of water to produce oxygen, the reverse process – the reduction of oxygen – also plays a role, albeit indirectly. Photosynthetic organisms, such as plants and algae, utilize the energy from sunlight to split water molecules, releasing oxygen as a byproduct. However, in certain circumstances, oxygen can be reduced back to water via processes like photorespiration, a pathway that can reduce the efficiency of photosynthesis under high-light conditions.
Industrial Significance
The reduction of oxygen to water is also central to various industrial applications. Fuel cells, for instance, utilize the electrochemical reduction of oxygen to generate electricity. This process is highly efficient and environmentally friendly, making fuel cells a promising alternative energy source. Furthermore, the controlled reduction of oxygen is crucial in many chemical manufacturing processes, preventing unwanted oxidation reactions and ensuring product purity.
Mechanisms of Oxygen Reduction
The reduction of O₂ to H₂O involves a series of intermediate steps, with the exact mechanism varying depending on the system.
Four-Electron Reduction
The overall balanced equation for the complete reduction of oxygen to water is:
O₂ + 4H⁺ + 4e⁻ → 2H₂O
This represents a four-electron reduction process. However, this doesn't happen in a single step. Instead, it involves several intermediate species, each with varying levels of reactivity and stability:
- Superoxide radical (O₂⁻): This is a one-electron reduction product of O₂, a highly reactive species that can cause significant cellular damage through oxidative stress.
- Hydrogen peroxide (H₂O₂): This is formed by the reduction of superoxide, also a reactive oxygen species (ROS) that can damage cellular components.
- Hydroxyl radical (•OH): Further reduction of hydrogen peroxide can lead to the highly reactive hydroxyl radical, another ROS that contributes to oxidative damage.
Enzymatic Reduction in Biology
In biological systems, the reduction of oxygen is predominantly catalyzed by enzymes to prevent the formation of harmful ROS. The most critical enzyme in this process is cytochrome c oxidase (COX), the terminal enzyme of the mitochondrial electron transport chain. COX efficiently catalyzes the four-electron reduction of O₂ to H₂O, minimizing the production of ROS. This enzyme utilizes a complex system of metal cofactors, including heme groups and copper ions, to facilitate the transfer of electrons and protons, ensuring a controlled and efficient reduction. Other enzymes, like superoxide dismutase (SOD) and catalase, play supporting roles by scavenging ROS produced during the process.
Non-Enzymatic Reduction
Outside biological systems, the reduction of oxygen can occur via non-enzymatic pathways. These pathways are often less controlled and can lead to the formation of ROS. For instance, the reduction of oxygen by transition metals, such as iron and copper, can produce superoxide and other ROS. These reactions can contribute to corrosion and the degradation of materials. In some industrial processes, the controlled reduction of oxygen is achieved using electrochemical methods or chemical reducing agents.
Factors Affecting Oxygen Reduction
Several factors influence the rate and efficiency of oxygen reduction:
pH
The pH of the environment plays a crucial role, as the reduction reaction involves protons (H⁺). A lower pH (more acidic conditions) increases the concentration of protons, facilitating the reduction.
Potential
The redox potential of the system influences the thermodynamics of the reaction. A higher redox potential favors the reduction of oxygen.
Catalysts
The presence of catalysts, such as enzymes or metal complexes, significantly affects the rate of oxygen reduction. Catalysts lower the activation energy, accelerating the reaction.
Temperature
Higher temperatures generally increase the rate of reaction, but excessive heat can also damage the catalyst or denature enzymes.
Oxygen Concentration
The rate of oxygen reduction is directly proportional to the concentration of oxygen. Higher oxygen partial pressures lead to faster reduction.
Implications of Inefficient Oxygen Reduction
Inefficient or uncontrolled reduction of oxygen can have significant consequences:
Oxidative Stress
The formation of ROS during incomplete oxygen reduction causes oxidative stress, which can damage cellular components like DNA, proteins, and lipids. Oxidative stress is implicated in various diseases, including cancer, aging, and neurodegenerative disorders.
Corrosion
In industrial settings, uncontrolled oxygen reduction can contribute to corrosion of metals, leading to material degradation and economic losses.
Environmental Impact
The incomplete reduction of oxygen can release harmful pollutants into the environment, contributing to air and water pollution.
Conclusion
The reduction of molecular oxygen to water is a critical process in both biological and chemical systems. While the overall reaction appears straightforward, the underlying mechanisms are intricate, involving several intermediate steps and potential byproducts. Understanding the factors that influence oxygen reduction and the consequences of inefficient reduction is crucial for various applications, ranging from optimizing energy production in fuel cells to preventing oxidative damage in biological systems. Future research continues to explore innovative methods to control and harness this fundamental reaction, contributing to advancements in various fields, from medicine to materials science. The ongoing exploration of oxygen reduction mechanisms and their impact will continue to reveal new insights and inspire further innovation. Further research into efficient and controlled oxygen reduction will be crucial in developing sustainable energy solutions and mitigating the detrimental effects of oxidative stress.
Latest Posts
Latest Posts
-
1 4 Inch Is How Many Mm
Mar 17, 2025
-
What Is A Diaphragm In Microscope
Mar 17, 2025
-
Sample Evidence Can Prove That A Null Hypothesis Is True
Mar 17, 2025
-
How Many Valence Electrons Does Calcium Ca Have
Mar 17, 2025
-
Common Multiples Of 4 5 6
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Process Reduces Molecular Oxygen To Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
