Which State Of Matter Generally Has The Highest Density
Kalali
Apr 01, 2025 · 5 min read
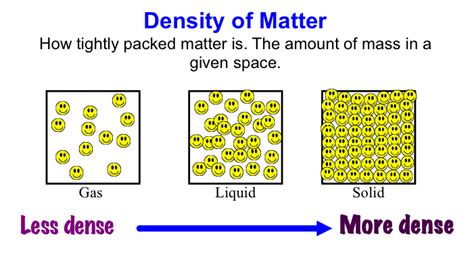
Table of Contents
Which State of Matter Generally Has the Highest Density?
The question of which state of matter boasts the highest density isn't a simple "solids always win" answer. While it's generally true that solids are denser than liquids and gases, there are exceptions and nuances that make this topic far more fascinating than a straightforward response might suggest. This comprehensive guide will explore the density of solids, liquids, and gases, delving into the underlying physics, exceptions, and real-world examples to provide a complete understanding.
Understanding Density: Mass vs. Volume
Before diving into the states of matter, let's define density. Density (ρ) is a fundamental physical property that describes how much mass is packed into a given volume. It's calculated as:
Density (ρ) = Mass (m) / Volume (V)
The units of density are typically grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). A higher density means more mass is squeezed into the same volume.
Solids: The Usual Suspects for High Density
Solids generally exhibit the highest densities compared to liquids and gases. This is because the constituent particles (atoms, ions, or molecules) are tightly packed together in a relatively fixed arrangement. The strong intermolecular forces hold the particles close, resulting in a smaller volume for a given mass.
Examples of High-Density Solids:
- Metals: Many metals, such as osmium and iridium, possess exceptionally high densities due to their tightly packed atomic structures and high atomic masses. Osmium, in fact, holds the title of the densest naturally occurring element.
- Ceramics: Certain ceramics, especially those with high atomic number components, can also display high densities.
- Crystalline Structures: The crystal structure plays a significant role. Close-packed arrangements, like those found in face-centered cubic (FCC) and hexagonal close-packed (HCP) structures, lead to higher densities compared to more open structures.
Factors Affecting Solid Density:
- Atomic Mass: Heavier atoms contribute to higher density.
- Atomic Radius: Smaller atoms allow for closer packing, increasing density.
- Crystal Structure: As mentioned, the arrangement of atoms in the crystal lattice significantly influences density.
- Temperature and Pressure: While generally less impactful than the above factors, temperature and pressure can slightly alter the density of solids through thermal expansion and compression.
Liquids: A Middle Ground
Liquids have a higher density than gases but generally a lower density than solids. This is because the particles in a liquid are closer together than in a gas but are not as rigidly arranged as in a solid. The particles can move around and slide past each other, leading to a less compact structure.
Exceptions to the Rule:
While solids typically reign supreme in density, some liquids can surprisingly exhibit higher density than certain solids. This often occurs when the solid has a less efficient packing structure, or its constituent particles have relatively low atomic mass.
Examples of High-Density Liquids:
- Mercury: This liquid metal has a significantly high density compared to most other liquids.
- Liquid Bromine: This halogen, although not as dense as mercury, is denser than many common liquids.
- Water (at Specific Temperatures): While not extremely dense, water displays anomalous behavior. Its maximum density occurs at 4°C (39.2°F), with ice being less dense than liquid water. This unique property is crucial for aquatic life survival.
Gases: The Least Dense State
Gases have the lowest densities among the three states of matter. The particles in a gas are widely dispersed and move rapidly and randomly, leading to a large volume for a given mass. The weak intermolecular forces allow for significant expansion and compressibility.
Factors Affecting Gas Density:
- Temperature: Higher temperatures increase the kinetic energy of gas particles, causing them to spread out and decrease density.
- Pressure: Higher pressure forces gas particles closer together, resulting in increased density.
- Molecular Weight: Gases with higher molecular weight will generally have higher density at a given temperature and pressure.
Examples of High-Density Gases (Relative to Other Gases):
Although gases are generally less dense than solids and liquids, some gases are denser than others. The concept of "high-density gas" is relative within this state of matter.
- Radon: As a noble gas with a high atomic mass, Radon is among the densest gases.
- Xenon: Another noble gas with a significant atomic mass, Xenon is considerably denser than lighter gases like helium or hydrogen.
- Sulfur Hexafluoride: This man-made gas has a relatively high molecular weight and therefore a higher density than many common atmospheric gases.
The Importance of Pressure and Temperature
It's crucial to remember that the density of any substance is dependent on temperature and pressure. Increasing pressure generally increases density, while increasing temperature typically decreases it (with some exceptions like water). Thus, direct comparisons of density between states require specifying the conditions under which the measurements are taken.
Extreme Conditions and Exotic States
At extreme pressures and temperatures, the distinctions between states of matter blur. Under these conditions, new states of matter can emerge, such as plasmas and Bose-Einstein condensates. The densities of these states can vary greatly depending on the specific conditions. Plasma, for example, can exhibit a wide range of densities, from relatively low to extremely high. Bose-Einstein condensates, on the other hand, achieve extraordinary densities, but are only achievable at extremely low temperatures.
Conclusion: The Density Championship
While solids generally exhibit the highest densities, the statement is not universally true. The interplay of atomic mass, atomic radius, crystal structure, temperature, and pressure significantly influences the density of any substance. Some liquids, especially those composed of heavy atoms, can surpass the density of some solids. The relative density of gases is much lower than that of solids and liquids. The most dense naturally occurring substance remains Osmium, a solid metal. However, the complexity of this seemingly simple question highlights the fascinating and multifaceted nature of the physical properties of matter under various conditions. The study of density isn't merely an academic pursuit; it's a vital concept for numerous scientific and engineering disciplines, impacting fields from materials science to astrophysics.
Latest Posts
Latest Posts
-
How Many Grams In 250 Mg
Apr 02, 2025
-
El Animal Mas Pequeno Del Mundo
Apr 02, 2025
-
A Limit Involving The Cosine Functio
Apr 02, 2025
-
How Many Cm Is 28 Inch
Apr 02, 2025
-
Rutherfords Gold Foil Experiment Determined That
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Generally Has The Highest Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
