Which State Of Matter Has The Most Energy
Kalali
Mar 12, 2025 · 5 min read
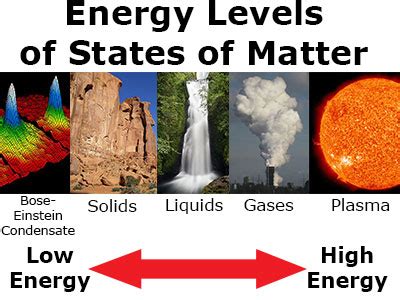
Table of Contents
Which State of Matter Has the Most Energy? Exploring Kinetic Energy and Phase Transitions
The question of which state of matter possesses the most energy is deceptively simple. It's not a matter of a single state always having the most energy, but rather a complex interplay of factors including temperature, type of substance, and the specific energy being considered (kinetic vs. potential). Let's delve into the fascinating world of thermodynamics and phase transitions to unravel this intriguing question.
Understanding States of Matter and Energy
Before we can determine which state holds the most energy, we need to establish a clear understanding of the three fundamental states (ignoring plasma for now): solid, liquid, and gas. Each state represents a different level of molecular kinetic energy:
Solids: The Lowest Energy State (Generally)
In solids, molecules are tightly packed in a rigid structure, exhibiting minimal movement beyond slight vibrations around their fixed positions. This limited motion translates to low kinetic energy. The molecules are held together by strong intermolecular forces, resulting in a defined shape and volume. Think of the tightly packed atoms in a crystal lattice – they barely move.
Liquids: Increased Kinetic Energy and Molecular Freedom
Liquids exhibit higher kinetic energy than solids. Molecules in a liquid are still relatively close together, but they have more freedom of movement. They can slide past each other, leading to a defined volume but an indefinite shape. This increased mobility stems from weaker intermolecular forces compared to solids, allowing for greater kinetic energy and molecular freedom.
Gases: The Highest Kinetic Energy State (Generally)
Gases possess the highest kinetic energy among the three common states of matter. Molecules in a gaseous state are widely dispersed, moving rapidly and randomly in all directions. The weak intermolecular forces allow for significant separation between molecules, resulting in an indefinite shape and volume. This high degree of molecular motion translates to significantly higher kinetic energy compared to solids and liquids. Imagine the rapid, chaotic movement of air molecules.
The Role of Temperature and Phase Transitions
Temperature is a crucial factor influencing the energy content of a substance. Temperature is a direct measure of the average kinetic energy of the molecules within a substance. Higher temperatures mean higher average kinetic energy, irrespective of the state of matter.
Phase transitions further complicate the picture. Consider the energy changes during transitions:
-
Melting (Solid to Liquid): Energy is absorbed to overcome the intermolecular forces holding the solid together, increasing the kinetic energy of the molecules and allowing them to transition to the liquid phase.
-
Vaporization (Liquid to Gas): A significant amount of energy is absorbed to overcome the remaining intermolecular forces, enabling molecules to escape into the gaseous phase. This is a process that requires considerable energy input.
-
Sublimation (Solid to Gas): This direct transition skips the liquid phase and requires even more energy input to overcome the strong intermolecular forces holding the solid together.
-
Conversely, phase transitions in the opposite direction (freezing, condensation, deposition) release energy.
This means that a gas at a low temperature might have less energy than a liquid at a high temperature. The energy depends not only on the state but also on the temperature.
Beyond the Basics: Considering Potential Energy
The discussion so far has focused primarily on kinetic energy, the energy associated with the motion of molecules. However, we must also consider potential energy, which is stored energy related to the position or configuration of molecules.
Potential energy plays a significant role in the energy content of a substance, particularly in the context of intermolecular forces. Stronger intermolecular forces imply higher potential energy. While solids generally have lower kinetic energy, they might possess higher potential energy due to strong intermolecular bonds.
This means that a solid at a very low temperature might have lower total energy (kinetic + potential) than a gas at a high temperature. The total energy is the sum of both kinetic and potential energies.
Plasma: The Fourth State of Matter
Plasma, often considered the fourth state of matter, represents a significantly higher energy state compared to solids, liquids, and gases. In plasma, atoms are ionized, meaning they have lost or gained electrons, resulting in a mixture of ions and free electrons. This ionization process requires a substantial input of energy, leading to extremely high kinetic energies for the charged particles. The behavior of plasma is dominated by electromagnetic forces, setting it apart from the other three states. Consider the sun – a giant ball of plasma with incredibly high energy.
Specific Examples and Considerations
Let's analyze specific examples to clarify the interplay between state, temperature, and energy:
-
Water: Water vapor (gas) at 100°C has higher kinetic energy than liquid water at 0°C. However, liquid water at 100°C might have higher total energy (kinetic and potential) than water vapor at, say, -10°C.
-
Iron: Solid iron at room temperature will have significantly less kinetic energy than gaseous iron at high temperatures. However, the potential energy in solid iron due to its strong metallic bonding may be substantial.
-
Helium: Helium, even as a gas at room temperature, still exhibits relatively weak intermolecular forces, resulting in lower potential energy. However, the kinetic energy is higher than a solid or liquid helium at the same temperature.
Conclusion: No Single "Most Energetic" State
There is no single definitive answer to the question of which state of matter has the most energy. The energy content depends on multiple factors including:
-
Temperature: Higher temperatures generally imply higher average kinetic energy, regardless of the state of matter.
-
Substance: The type of substance greatly influences the strength of intermolecular forces, impacting potential energy.
-
Energy type: The total energy is the sum of kinetic and potential energies. One might outweigh the other depending on the specific conditions.
Therefore, a gas at high temperatures generally has higher kinetic energy than a solid or liquid at low temperatures. However, a solid with strong intermolecular forces might have higher total energy than a gas at low temperatures, due to the significant potential energy stored in its bonds. The question requires careful consideration of temperature, the specific substance, and both kinetic and potential energy contributions. Understanding phase transitions and their associated energy changes is essential in grasping the nuances of this topic.
Latest Posts
Latest Posts
-
What Is Another Name For Condensation Reaction
Mar 12, 2025
-
How Are Elements Arranged In The Modern Periodic Table
Mar 12, 2025
-
How Do You Increase Charge Of A Capacitor
Mar 12, 2025
-
Cuanto Son 32 Onzas En Litros
Mar 12, 2025
-
What Is 3 As A Percentage Of 25
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has The Most Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
