Will There Be A Curve On Water Vapor Pressure
Kalali
Mar 31, 2025 · 6 min read
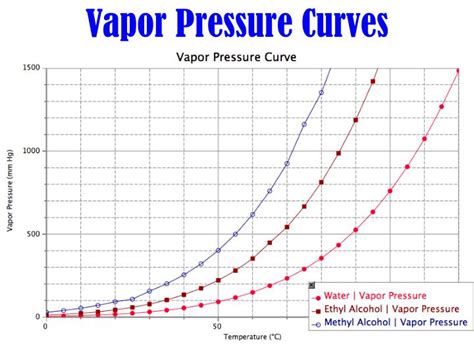
Table of Contents
Will There Be a Curve on Water Vapor Pressure? Exploring the Relationship Between Temperature and Vapor Pressure
Water vapor pressure, a crucial element in meteorology, atmospheric science, and numerous industrial processes, describes the pressure exerted by water vapor in a system. Understanding its behavior, particularly its relationship with temperature, is fundamental to predicting weather patterns, designing efficient industrial processes, and comprehending various environmental phenomena. This article delves into the complex relationship between temperature and water vapor pressure, addressing the question: will there be a curve on water vapor pressure? The answer, as we will explore, is a resounding yes, and understanding the nature of this curve is key to unlocking a deeper understanding of the atmospheric and environmental processes it governs.
The Fundamental Relationship: Temperature and Saturation Vapor Pressure
The relationship between temperature and water vapor pressure isn't linear; it's inherently non-linear, resulting in a characteristic curve. This is because the pressure exerted by water vapor is directly linked to the kinetic energy of water molecules. As temperature increases, the kinetic energy of water molecules also increases, leading to a greater number of molecules transitioning from the liquid or solid phase to the gaseous phase (evaporation or sublimation). This increased number of water molecules in the gaseous phase directly translates to a higher water vapor pressure.
However, the relationship is not simply proportional. The rate at which water molecules transition to the gaseous phase accelerates non-linearly with increasing temperature. This is due to the complex interactions between water molecules and the energy barriers they must overcome to escape the liquid or solid phase. Therefore, a small increase in temperature at lower temperatures can lead to a proportionally larger increase in vapor pressure compared to a similar temperature increase at higher temperatures.
This non-linear relationship is captured by the Clausius-Clapeyron equation, a fundamental equation in thermodynamics that describes the relationship between the vapor pressure of a substance and its temperature. While the equation itself isn't readily intuitive, it mathematically expresses the exponential nature of the curve we observe.
Understanding the Saturation Vapor Pressure Curve
The curve representing the relationship between temperature and saturation vapor pressure is not just any curve; it’s a specific curve with distinct characteristics. The curve is exponential, meaning the vapor pressure increases exponentially with temperature. This is vividly illustrated by the steep rise of the curve at higher temperatures.
Key Characteristics of the Saturation Vapor Pressure Curve:
- Exponential Growth: As mentioned, the curve shows an exponential increase in saturation vapor pressure with rising temperature. This means that for each degree Celsius increase in temperature, the increase in vapor pressure becomes progressively larger.
- Asymptotic Behavior: While the curve never truly reaches a horizontal asymptote (it continues to increase indefinitely), the rate of increase slows down at extremely high temperatures.
- Temperature Dependence: The curve is highly sensitive to temperature changes, particularly at lower temperatures. A small change in temperature can significantly impact the saturation vapor pressure.
- Dependence on Phase: The curve is also affected by the phase of water. The saturation vapor pressure curve for ice is different from that of liquid water. The sublimation curve (ice to vapor) lies below the evaporation curve (liquid to vapor).
Factors Influencing the Water Vapor Pressure Curve
While temperature is the primary driver of the water vapor pressure curve, other factors can influence its shape and position:
- Altitude: At higher altitudes, the atmospheric pressure is lower. This lower pressure allows for a higher saturation vapor pressure at any given temperature. Essentially, the curve shifts upwards at higher altitudes.
- Pressure: As mentioned above, changes in total atmospheric pressure directly impact the saturation vapor pressure. Reduced pressure leads to higher saturation vapor pressure, and increased pressure leads to lower saturation vapor pressure.
- Presence of other gases: The presence of other gases in the air, such as pollutants, can influence the saturation vapor pressure, though typically to a lesser extent than temperature and pressure. These gases can interact with water molecules, slightly altering their behavior and thus affecting vapor pressure.
- Humidity: While relative humidity doesn't directly affect the curve itself, it represents the ratio of the actual vapor pressure to the saturation vapor pressure at a given temperature. High relative humidity indicates that the air is close to saturation, meaning the actual vapor pressure is nearing the saturation vapor pressure indicated on the curve.
Practical Applications and Significance of the Curve
Understanding the water vapor pressure curve is crucial in various fields:
- Meteorology: Meteorologists use the curve to understand and predict weather patterns. Factors like dew point, relative humidity, and cloud formation are all intricately linked to the saturation vapor pressure curve. The curve helps predict the likelihood of precipitation, fog formation, and other weather events.
- Climate Science: The curve plays a key role in understanding climate change. As global temperatures increase, the atmosphere's capacity to hold water vapor also increases, leading to more intense rainfall events and potentially exacerbating the effects of climate change.
- HVAC Systems: The principles governing the saturation vapor pressure curve are fundamental in the design and operation of heating, ventilation, and air conditioning (HVAC) systems. Understanding the relationship between temperature and humidity is crucial for creating comfortable and efficient indoor environments.
- Industrial Processes: Many industrial processes, such as drying, humidification, and distillation, rely on a precise control of water vapor pressure. The curve provides a framework for optimizing these processes and improving their efficiency.
- Agriculture: Water vapor pressure significantly impacts plant growth and development. Understanding the curve is essential for optimizing irrigation practices and ensuring healthy crop yields.
Beyond the Curve: Relative Humidity and its Implications
While the saturation vapor pressure curve illustrates the maximum amount of water vapor the air can hold at a given temperature, the actual amount of water vapor in the air is described by relative humidity. Relative humidity is the percentage of saturation vapor pressure currently achieved at a given temperature. For example, if the saturation vapor pressure at 25°C is 31.6 mbar and the actual vapor pressure is 15.8 mbar, then the relative humidity is 50%.
The Interplay of Temperature, Saturation Vapor Pressure, and Relative Humidity
Changes in temperature significantly affect both saturation vapor pressure and relative humidity. Consider this scenario: if warm, humid air cools down, the saturation vapor pressure decreases, while the actual water vapor content remains the same (at least initially). This leads to an increase in relative humidity. If the air continues to cool, eventually the relative humidity reaches 100%, and condensation occurs, leading to dew or cloud formation. This highlights the importance of understanding the interplay between temperature, saturation vapor pressure, and relative humidity in numerous environmental and meteorological processes.
Conclusion: The Indispensable Water Vapor Pressure Curve
The relationship between temperature and water vapor pressure is not simply linear; it is governed by a complex, non-linear curve. This curve, primarily described by the Clausius-Clapeyron equation, is an exponential function, reflecting the increasing capacity of the atmosphere to hold water vapor as temperature rises. Understanding the characteristics of this curve is essential for making accurate weather predictions, comprehending climate change impacts, designing efficient industrial processes, optimizing agricultural practices, and gaining a deeper understanding of various environmental phenomena. The water vapor pressure curve, therefore, remains a fundamental concept in numerous scientific and technological disciplines. Its implications are far-reaching, shaping our understanding of the world around us and guiding technological advancements across various sectors. From predicting the next rainfall to designing more efficient air conditioning systems, the impact of this seemingly simple curve is profound and wide-ranging.
Latest Posts
Latest Posts
-
17 Of 20 Is What Percent
Apr 01, 2025
-
What Is 6 To The Power Of 3
Apr 01, 2025
-
Cual Es El 10 Por Ciento De 1000
Apr 01, 2025
-
What Is 11 15 As A Percent
Apr 01, 2025
-
Un Triangulo Equilatero Es Tambien Un Triangulo
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Will There Be A Curve On Water Vapor Pressure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
