How Many Valence Electrons Does Halogens Have
Kalali
Mar 27, 2025 · 5 min read
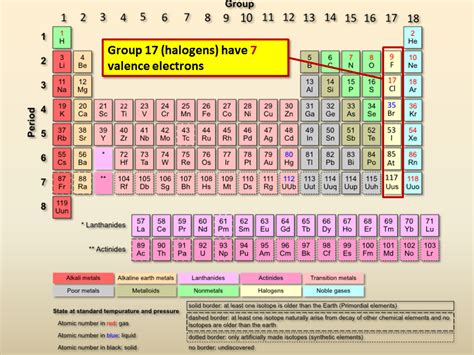
Table of Contents
How Many Valence Electrons Do Halogens Have? A Deep Dive into Group 17
The halogens, a vibrant and reactive group of nonmetals found in Group 17 (VIIA) of the periodic table, hold a special place in chemistry. Their unique properties, largely dictated by their electron configuration, make them crucial in various applications, from everyday life to cutting-edge technologies. Understanding their valence electrons is key to unlocking this understanding. So, how many valence electrons do halogens have? The answer is a consistent seven. This article will delve into the details, explaining why this number is so important and exploring its implications on their reactivity and properties.
Understanding Valence Electrons
Before we dive into the specifics of halogens, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely held and therefore play the most significant role in chemical bonding and reactions. They determine an element's reactivity and the types of bonds it can form. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outer shell – a state often referred to as the "octet rule" (eight electrons in the outer shell). Noble gases, with their full outer shells, are famously unreactive because they have no tendency to gain or lose electrons.
The Electron Configuration of Halogens
The halogens—fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)—all share a common characteristic: they have seven valence electrons. This consistent electron configuration is the defining feature of this group and is responsible for their similar chemical behavior.
Let's examine the electron configuration of each halogen:
- Fluorine (F): 1s²2s²2p⁵ (2+5=7 valence electrons)
- Chlorine (Cl): 1s²2s²2p⁶3s²3p⁵ (2+5=7 valence electrons)
- Bromine (Br): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵ (2+5=7 valence electrons)
- Iodine (I): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁵ (2+5=7 valence electrons)
- Astatine (At): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁵ (2+5=7 valence electrons)
Notice how the outermost shell (the highest principal quantum number 'n') always contains seven electrons (two in the s-subshell and five in the p-subshell). This explains why they all possess seven valence electrons. The inner shells are filled, representing core electrons that do not actively participate in chemical bonding.
The Significance of Seven Valence Electrons: Reactivity and Bonding
The presence of seven valence electrons significantly impacts the chemical behavior of halogens. Because they are only one electron short of a stable octet, halogens are highly reactive. They readily gain one electron to form a stable anion with a -1 charge (halide ion). This tendency explains their strong electronegativity, meaning they have a high attraction for electrons in a chemical bond.
This electron-gaining tendency leads to several key characteristics:
-
Formation of Ionic Compounds: Halogens readily react with metals, which tend to lose electrons easily. The transfer of one electron from a metal atom to a halogen atom forms an ionic bond, creating an ionic compound. For example, sodium chloride (NaCl), or common table salt, is formed by the transfer of an electron from sodium (Na) to chlorine (Cl).
-
Formation of Covalent Compounds: Halogens can also form covalent bonds with other nonmetals by sharing electrons. However, even in these bonds, the halogens tend to attract the shared electrons more strongly due to their high electronegativity. This leads to polar covalent bonds, where there's an uneven distribution of electron density.
-
Oxidizing Agents: Due to their high electronegativity and tendency to gain electrons, halogens act as powerful oxidizing agents. They readily accept electrons from other substances, causing them to be oxidized (lose electrons). This property is widely exploited in various chemical processes and applications.
Halogens in Everyday Life and Beyond
The unique properties stemming from their seven valence electrons make halogens crucial in many aspects of our lives:
-
Fluorine: Fluorine is essential in preventing tooth decay (fluoridation of water), and various fluorocarbons are used in refrigerants (though many are being phased out due to environmental concerns).
-
Chlorine: Chlorine is widely used in water purification to disinfect drinking water and swimming pools. It's also a vital component in many industrial processes and the production of plastics (polyvinyl chloride or PVC).
-
Bromine: Bromine is used in flame retardants, agricultural chemicals, and dyes.
-
Iodine: Iodine is an essential nutrient, crucial for thyroid hormone production, and is often added to table salt to prevent iodine deficiency.
-
Astatine: Astatine is a radioactive element, and its applications are limited due to its rarity and radioactivity. However, it holds research interest in nuclear medicine.
Conclusion: Seven Valence Electrons – The Key to Halogen Chemistry
The consistent presence of seven valence electrons in halogens is the defining feature of this fascinating group of elements. This electron configuration dictates their high reactivity, strong electronegativity, and propensity to form -1 charged anions. Understanding this fundamental characteristic unlocks an appreciation for their widespread applications, from everyday uses like water purification and dental health to more specialized roles in various industries and research. Their significance in various chemical reactions and their unique properties underscore the importance of understanding the fundamental relationship between electron configuration and chemical behavior. The seven valence electrons are, without a doubt, the key to understanding the captivating chemistry of the halogens. Further exploration into the specific reactions and applications of each halogen would provide an even deeper understanding of this important group of elements and their vital roles in our world.
Latest Posts
Latest Posts
-
What Is Freezing In Celsius Degrees
Mar 30, 2025
-
Any Set Of Ordered Pairs Is Called A
Mar 30, 2025
-
What Percentage Is 11 Out Of 20
Mar 30, 2025
-
Cuanto Es 2 Kilos En Libras
Mar 30, 2025
-
3 Is What Percent Of 8
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Halogens Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
