V2 Co3 3 Cation And Anion
Kalali
Mar 30, 2025 · 5 min read
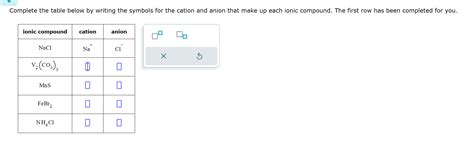
Table of Contents
V2(CO3)3: Delving into the Cation and Anion of Vanadium(III) Carbonate
Vanadium(III) carbonate, with the chemical formula V₂(CO₃)₃, presents a fascinating case study in inorganic chemistry. Understanding its cation and anion components is crucial for comprehending its properties, synthesis, and potential applications. This detailed exploration will delve into the intricacies of V³⁺ (the vanadium(III) cation) and CO₃²⁻ (the carbonate anion), highlighting their individual characteristics and how they interact to form V₂(CO₃)₃.
Understanding the Vanadium(III) Cation (V³⁺)
The vanadium(III) cation, V³⁺, is a transition metal ion with a unique electronic configuration that dictates its chemical behavior. Let's break down its key features:
Electronic Configuration and Oxidation State
Vanadium, with atomic number 23, possesses the electronic configuration [Ar] 3d³ 4s². In the V³⁺ ion, three electrons are lost, resulting in the configuration [Ar] 3d². This 3+ oxidation state is relatively stable for vanadium, although it can exist in other oxidation states (+2, +4, +5) as well. The presence of three unpaired d electrons contributes to the paramagnetic nature of V³⁺, meaning it is attracted to magnetic fields.
Coordination Chemistry and Complex Formation
V³⁺ is a hard Lewis acid, meaning it prefers to coordinate with hard Lewis bases, such as oxygen-containing ligands. It readily forms coordination complexes with a variety of ligands, often exhibiting coordination numbers of 6 (octahedral geometry) or 4 (tetrahedral geometry). The specific geometry depends on the steric factors of the ligands and the overall crystal field stabilization energy (CFSE). The formation of these complexes significantly influences the reactivity and solubility of vanadium(III) compounds.
Reactivity and Redox Properties
V³⁺ exhibits a propensity to undergo redox reactions, readily acting as both a reducing agent (losing electrons) and an oxidizing agent (gaining electrons). Its ability to exist in multiple oxidation states makes it a versatile component in various redox processes. However, in the context of V₂(CO₃)₃, its stability in the +3 oxidation state is crucial for the compound's overall structure and properties.
Exploring the Carbonate Anion (CO₃²⁻)
The carbonate anion, CO₃²⁻, is a ubiquitous polyatomic ion found in various inorganic and organic compounds. Its structure and bonding contribute to its distinctive properties:
Structure and Bonding
The carbonate ion has a trigonal planar geometry, with the carbon atom at the center and the three oxygen atoms surrounding it. Each carbon-oxygen bond possesses partial double bond character due to resonance, leading to an equal bond length between the carbon and each oxygen atom. This resonance stabilization contributes to the stability of the carbonate anion.
Reactivity and Acid-Base Properties
CO₃²⁻ is a weak diprotic base, meaning it can accept two protons (H⁺). Its first deprotonation produces the bicarbonate ion (HCO₃⁻), and the second deprotonation yields carbonic acid (H₂CO₃), which readily decomposes into water and carbon dioxide. This amphoteric nature is crucial in various chemical reactions and natural processes.
Complex Formation and Coordination Chemistry
The carbonate ion can act as a ligand, forming coordination complexes with various metal ions. Its ability to coordinate through one or more oxygen atoms allows it to bridge metal centers, forming polymeric or oligomeric structures. In V₂(CO₃)₃, the carbonate ion acts as a bridging ligand, connecting the vanadium(III) cations.
The Synthesis and Properties of V₂(CO₃)₃
Understanding the individual properties of V³⁺ and CO₃²⁻ allows us to appreciate the characteristics of V₂(CO₃)₃. While the exact synthesis methods and precise properties may vary depending on the reaction conditions, some common features emerge:
Synthesis Strategies
The synthesis of V₂(CO₃)₃ often involves reacting a vanadium(III) salt, such as VCl₃ or V₂(SO₄)₃, with a carbonate source, such as Na₂CO₃ or K₂CO₃. The reaction conditions, including pH, temperature, and concentration, are crucial in obtaining the desired product. Controlling these parameters helps in minimizing the formation of competing vanadium(III) hydroxides or oxides.
Physical Properties
V₂(CO₃)₃ is typically a solid compound. Its exact color and crystallinity may depend on the synthesis route and the presence of impurities. It's generally insoluble in water, reflecting the relatively low solubility of many metal carbonates. However, its solubility can be influenced by the pH of the solution and the presence of complexing agents.
Chemical Properties
As a metal carbonate, V₂(CO₃)₃ is susceptible to acid decomposition. Reacting it with strong acids will liberate carbon dioxide and form soluble vanadium(III) salts. It may also undergo thermal decomposition at elevated temperatures, possibly yielding vanadium oxides and carbon dioxide. Its reactivity with oxidizing agents needs careful consideration, as it may lead to higher oxidation states of vanadium.
Applications and Importance of V₂(CO₃)₃
While V₂(CO₃)₃ may not have widespread commercial applications yet, its unique properties make it a subject of ongoing research. Its potential applications could stem from:
Precursor for Other Vanadium Compounds
V₂(CO₃)₃ can potentially act as a precursor for the synthesis of other vanadium compounds. Its controlled decomposition or reaction with other reagents could lead to the preparation of other vanadium-containing materials with desired properties.
Catalytic Applications
The presence of vanadium in various oxidation states and the availability of carbonate ligands suggest potential applications in catalysis. Further research could explore its catalytic activity in various organic and inorganic reactions.
Material Science Applications
Vanadium compounds, including carbonates, are being investigated for their potential applications in various material science fields. This could include the development of novel composite materials, coatings, or functional materials.
Biological Significance
While not widely studied, the potential biological effects of vanadium(III) compounds warrants further investigation. Understanding the interactions between V₂(CO₃)₃ and biological systems could lead to applications in medicine or environmental science.
Conclusion
V₂(CO₃)₃, with its distinct vanadium(III) cation and carbonate anion components, offers a rich landscape for investigation in chemistry and materials science. A deeper understanding of its synthesis, properties, and reactivity is crucial in exploring its potential applications across various fields. Further research is needed to fully unlock the potential of this intriguing compound. The insights gained from studying V₂(CO₃)₃ will enhance our understanding of transition metal chemistry, coordination chemistry, and the design of novel materials with tailored properties. The continued study of this compound promises to yield exciting discoveries and potential applications in the future. The exploration of its reactivity with different ligands and under various conditions holds the key to unlocking its full potential and its contributions to various scientific advancements.
Latest Posts
Latest Posts
-
What Is 6 To The Power Of 3
Apr 01, 2025
-
Cual Es El 10 Por Ciento De 1000
Apr 01, 2025
-
What Is 11 15 As A Percent
Apr 01, 2025
-
Un Triangulo Equilatero Es Tambien Un Triangulo
Apr 01, 2025
-
What Are The Inner Transition Metals
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about V2 Co3 3 Cation And Anion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
